Abstract
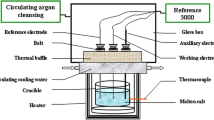
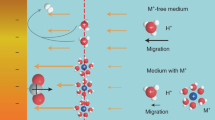
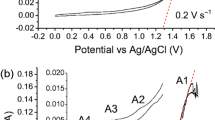
The cathodic behavior of molten CaCl2, CaCl2-CaO and equimolar CaCl2-NaCl-CaO was studied by cyclic voltammograms and constant potential polarization at temperatures of 1123 to 1173 K on molybdenum and titanium electrodes. The diffusion coefficient of Ca2+ (CaO) in molten CaCl2-CaO was calculated from the linear relationship between the square root of scan rate and the peak current density. The deposition potentials and the potential temperature coefficient of CaO in molten CaCl2-0.5mol%CaO and CaCl2-NaCl-0.5mol%CaO were also obtained from their cyclic voltammograms. The result shows that CaO is more easily reduced than CaCl2. The addition of NaCl in molten CaCl2-CaO induces the underpotential electrodeposition of CaO.
Similar content being viewed by others
References
T.H. Okabe, M. Nakamura, T. Oishi, and K. Ono, Electrochemical deoxidation of titanium, Metall. Trans. B, 24(1993), p.449.
C.Z. Chen, D.J. Fray, and T.W. Farthing, Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride, Nature, 407(2000), p.361.
K. Ono and R.O. Suzuki, A new concept for producing Ti sponge: calciothermic reduction, JOM, 54(2002), p.59.
S.Q. Jiao and H.M. Zhu, Novel metallurgical process for titanium production, J. Mater. Res., 21(2006), p.2172.
G.Z. Chen, E. Gordo, and D.J. Fray, Direct electrolytic preparation of chromium powder, Metall. Mater. Trans. B, 35(2004), p.223.
M.F. Liu, Z.C. Guo, and W.C. Lu, An investigation into electrochemical reduction of TiO2 pellet, Trans. Inst. Min. Metall. Sect. C, 114(2005), p.87.
D.H. Wang, G.H. Qiu, X.B. Jin, et al., Electrochemical metallization of solid terbium oxide, Angew. Chem. Int. Ed., 45(2006), p.2384.
J.H. Du, Z.P. Xi, Q.Y. Li, et al., Effect of TiO2 cathode performance on preparation of Ti by electro-deoxidation, Trans. Nonferrous Met. Soc. China, 17(2007), p.s514.
X.L. Guo, Z.C. Guo, and Z. Wang, Direct preparation of TiFe alloy by electrolytic reduction from TiO2 and Fe2O3, J. Univ. Sci. Technol. Beijing (in Chinese), 30(2008), No.6, p.620.
C. Schwandt, D.T.L. Alexander, and D.J. Fray, The electro-deoxidation of porous titanium dioxide precursors in molten calcium chloride under cathodic potential control, Electrochem. Acta, 54(2009), p.3819
S.L. Wang and Y.J. Li, Reaction mechanism of direct electrochemical reduction of titanium dioxide in molten calcium chloride, J. Electroanal. Chem., 571(2004), p.37.
C. Schwandt and D.J. Fray, Determination of the kinetic pathway in the electrochemical reduction of titanium dioxide in molten calcium chloride, Electrochem. Acta, 51(2005), p.66.
D.T.L. Alexander, C. Schwandt, and D.J. Fray, Microstructural kinetics of phase transformations during electrochemical reduction of titanium dioxide in molten calcium chloride, Acta Mater., 54(2006), p.2933.
E.B. Freydina and D.J. Fray, Synthesis of Pb-Ca alloys by electrolysis of CaO in molten salts, Trans. Inst. Min. Metall. Sect. C, 111(2002), p.C79.
G.Z. Chen and D.J. Fray, Voltammetric studies of the oxygen-titanium binary system in molten calcium chloride, J. Electrochem. Soc., 249(2002), p.E455.
M. Mohamedi, B. Borresen, G.M. Haarberg, and R.J. Tunold, Anodic behavior of carbon electrodes in CaO-CaCl2 melts at 1123 K, J. Electrochem. Soc., 146(1999), p.1472.
W.J. Me, S.Z. Duan, and D. Inman, The cathodic reduction of carbon from LiCl-KCl-NaCl molten salt, Chem. J. Chin. Univ., 9(1998), p.547.
D.J. Fray, Use of thermodynamics and electrochemistry in understanding novel molten salt electrochemical process, [in] The 16th Iketani Conference, Tokyo, 2006, p.269.
E.M. Levin, C.R. Robbins, and H.F. McMurdie, Phase Diagrams for Ceramists, The American Ceramic Society, Columbus, Ohio, 1969, p.443.
T. Berzins and P. Delahay, Oscillographic polarographic waves for the reversible of metals on solid electrodes, J. Am. Chem. Soc., 36(1953), p.555.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the National Natural Science Foundation of China (No.50674027).
Rights and permissions
About this article
Cite this article
Wang, Sl., Wang, W., Li, Sc. et al. Cathodic behavior of molten CaCl2-CaO and CaCl2-NaCl-CaO. Int J Miner Metall Mater 17, 791–794 (2010). https://doi.org/10.1007/s12613-010-0391-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-010-0391-8