Abstract
Introduction
Cadmium (Cd) produces severe oxidative stress, which can result in serious clinical consequences and tissue injury. The aim of the present survey was to investigate the protective effects of native Iranian probiotics (Lactobacillus rhamnosus, L. helveticus, and L. casei) against cadmium (Cd)-induced toxicity against the small intestine and lung at histopathological and biochemical levels.
Materials and Methods
Twenty-one adult male Wistar rats were randomized into three groups of seven rats (control, Cd-treated (3 mg/kg), and concomitant Cd and mix probiotic treatment for 30 days). Histological alterations were appraised via hematoxylin & eosin, Trichrome Masson, and PAS staining. The qRT-PCR technique was applied to assess the expression of pro-apoptotic, anti-apoptotic, and pro-inflammatory genes. Antioxidant enzymes activity was measured via ZellBio kits.
Results
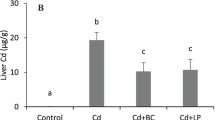
Probiotic-treated rats displayed low production of lipid peroxides, reduced malondialdehyde (MDA) level, and elevated contents of superoxide dismutase (SOD) and catalase (CAT) enzymes compared with Cd-treated rats. The results of qRT-PCR demonstrated the up-regulation of Bax, p53, and caspase 3 and down-regulation of Bcl2, TNF-α, and IL-6 genes in both the intestine and lungs of mix probiotic-treated rats compared with Cd-treated animals. Histopathological findings revealed that the probiotic formulation improved Cd-triggered tissue damage in the intestine and lungs.
Conclusion
The strong cytoprotective benefits of Iranian probiotics against Cd-induced tissue injury observed in this study may be due to their anti-inflammatory and antioxidant properties. Therefore, additional clinical and experimental research is required to explain the precise mechanisms of probiotics’ beneficial impacts and underline their potential therapeutic use.








Similar content being viewed by others
Availability of Data and Material
All data are included in the text; however, the raw data of this article will be made available by the authors, without undue reservation, to any qualified researcher.
References
Genchi G, Sinicropi MS, Lauria G et al (2020) The effects of cadmium toxicity. Int J Environ Res Public Health 17:1–24. https://doi.org/10.3390/ijerph17113782
Oguzturk H, Ciftci O, Aydin M et al (2012) Ameliorative effects of curcumin against acute cadmium toxicity on male reproductive system in rats.Andrologia. 243–249. https://doi.org/10.1111/j.1439-0272.2012.01273.x
Arroyo VS, Flores KM, Ortiz LB et al (2012) Liver and cadmium toxicity. J Drug Metab Toxicol S5:001.1–7 https://doi.org/10.4172/2157-7609.S5-001
Satarug S, Vesey DA, Gobe GC (2017) Kidney Cadmium Toxicity. Diabetes and High Blood Pressure : The Perfect Storm. https://doi.org/10.1620/tjem.241.65.Correspondence
Guo Y, Lam PK, Lam JCW, Zhou B (2015) Bioconcentration and Transfer of the Organophorous Flame Retardant 1, 3-dichloro 2-propyl phosphate ( TDCPP ) Causes Thyroid Endocrine Disruption and Developmental Neurotoxicity in Zebrafish Larvae. Environ Sci Technol 49:5123–5132. https://doi.org/10.1021/acs.est.5b00558
Simsek N, Karadeniz A, Kalkan Y et al (2009) Spirulina platensis feeding inhibited the anemia- and leucopenia-induced lead and cadmium in rats 164:1304–1309. https://doi.org/10.1016/j.jhazmat.2008.09.041
Kundu S, Sengupta S, Chatterjee S et al (2009) Cadmium induces lung inflammation independent of lung cell proliferation : a molecular approach 15:1–15. https://doi.org/10.1186/1476-9255-6-19
Wiley J (2008) Cadmium-induced neurological disorders : prophylactic role of taurine. 974–986. https://doi.org/10.1002/jat
Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia AA (2017) Cadmium toxicity and treatment: An update. Casp J Intern Med 8:135–145. https://doi.org/10.22088/cjim.8.3.135
Lestari S, Lestari P, Sciences N (2021) http://www.jmolekul.com. 16:202–209. https://doi.org/10.20884/1.jm.2021.16.3.775
Hossein-khannazer N, Azizi G, Eslami S et al (2019) The effects of cadmium exposure in the induction of inflammation. Immunopharmacol Immunotoxicol 0:1–8. https://doi.org/10.1080/08923973.2019.1697284
Muradoglu F, Gundogdu M, Ercisli S et al (2015) Cadmium toxicity affects chlorophyll a and b content , antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol Res 48(11): 1-7. https://doi.org/10.1186/s40659-015-0001-3
Eneman JD, Potts RJ, Osier M et al (2000) Suppressed oxidant-induced apoptosis in cadmium adapted alveolar epithelial cells and its potential involvement in cadmium carcinogenesis 147:215–228. https://doi.org/10.1016/s0300-483x(00)00215-8
Huang Y, Xia M, Wang H et al (2014) Cadmium selectively induces MIP-2 and COX-2 through PTEN-mediated Akt activation in RAW264. 7 cells. Toxicol Sci 138:310–321. https://doi.org/10.1093/toxsci/kfu013
Popov Aleksandrov A, Mirkov I, Tucovic D et al (2021) Cadmium and immunologically-mediated homeostasis of anatomical barrier tissues. Toxicol Lett 337:38–45. https://doi.org/10.1016/j.toxlet.2020.11.008
Phuagkhaopong S, Ospondpant D, Kasemsuk T, Vivithanaporn P (2017) are mediated by MAPK and NF- k B pathways. Neurotoxicology 60:82–91. https://doi.org/10.1016/j.neuro.2017.03.001
Tinkov AA, Gritsenko VA, Skalnaya MG et al (2018) Gut as a target for cadmium toxicity. Environ Pollut 235:429–434. https://doi.org/10.1016/j.envpol.2017.12.114
Feng P, Ye Z, Kakade A et al (2019) A review on gut remediation of selected environmental contaminants: possible roles of probiotics and gut microbiota. Nutrients 11:22. https://doi.org/10.3390/nu11010022
Zheng HJ, Guo J, Jia Q et al (2019) The effect of probiotic and synbiotic supplementation on biomarkers of inflammation and oxidative stress in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 142:303–313. https://doi.org/10.1016/j.phrs.2019.02.016
Śliżewska K, Markowiak-Kopeć P, Śliżewska W (2021) The role of probiotics in cancer prevention. Cancers (Basel) 13:1–22. https://doi.org/10.3390/cancers13010020
Shi LH, Balakrishnan K, Thiagarajah K et al (2016) Beneficial properties of probiotics. Trop Life Sci Res 27:73–90. https://doi.org/10.21315/tlsr2016.27.2.6
Novik G, Savich V (2020) Beneficial microbiota. Probiotics and pharmaceutical products in functional nutrition and medicine. Microbes Infect 22:8–18. https://doi.org/10.1016/j.micinf.2019.06.004
Vallianou N, Stratigou T, Christodoulatos GS et al (2020) Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep 9:179–192. https://doi.org/10.1007/s13679-020-00379-w
Feng T, Wang J (2020) Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic : a systematic review. Gut Microbes 12:1801944. https://doi.org/10.1080/19490976.2020.1801944
Wang Y, Wu Y, Wang Y et al (2017) Antioxidant properties of probiotic bacteria. Nutrients 9:521. https://doi.org/10.3390/nu9050521
Albano C, Silvetti T, Brasca M (2020) Screening of lactic acid bacteria producing folate and bio-enrichment. 1–9. https://doi.org/10.1093/femsle/fnaa059
Zhao J, Yu L, Zhai Q et al (2020) E ff ects of probiotic administration on hepatic antioxidative parameters depending on oxidative stress models : A meta-analysis of animal experiments. J Funct Foods 71:103936. https://doi.org/10.1016/j.jff.2020.103936
Chater S, Douki T, Garrel C et al (2008) Cadmium-induced oxidative stress and DNA damage in kidney of pregnant female rats. Comptes Rendus - Biol 331:426–432. https://doi.org/10.1016/j.crvi.2008.03.009
Keshtmand Z, Akbaribazm M, Bagheri Y, Oliaei R (2021) The ameliorative effects of Lactobacillus coagulans and Lactobacillus casei probiotics on CCl4-induced testicular toxicity based on biochemical, histological and molecular analyses in rat. Andrologia 53:1–10. https://doi.org/10.1111/and.13908
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C T method 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Foot NC (1933) The masson trichrome staining methods in routine laboratory use. Biotech Histochem 8:101–110. https://doi.org/10.3109/10520293309116112
Livak KJ, Schmittgen TD (2023) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt C T method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Huang Z, Wang Y, Qiu M et al (2019) Effects of T-2 toxin on digestive enzyme activity, intestinal histopathology and growth in shrimp Litopenaeus vannamei. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-49004-4
Saini S, Dhania G (2020) Cadmium as an environmental pollutant: ecotoxicological effects, health hazards, and bioremediation approaches for its detoxification from contaminated sites. In: Bioremediation of Industrial Waste for Environmental Safety. pp 357–387. https://doi.org/10.1007/978-981-13-3426-9_15
Camkurt MA, Ebru F, Bakacak M et al (2017) Evaluation of malondialdehyde, superoxide dismutase and catalase activity in fetal cord blood of depressed mothers. Clin Psychopharmacol Neurosci 15:35–39. https://doi.org/10.9758/cpn.2017.15.1.35
Apiamu A, Ogheneovo S (2021) Zinc-cadmium interactions instigated antagonistic alterations in lipid peroxidation, ascorbate peroxidase activity and chlorophyll synthesis in Phaseolus vulgaris leaves. Sci African 11:e00688. https://doi.org/10.1016/j.sciaf.2020.e00688
Whanger PD (1985) Metabolic interactions of selenium with cadmium, mercury, and silver. Adv Nutr Res 7:221–250. https://doi.org/10.1007/978-1-4613-2529-1_9
Haouem S, El HA (2013) Effect of cadmium on lipid peroxidation and on some antioxidants in the liver, kidneys and testes of rats given diet containing cadmium-polluted radish bulbs. J Toxicol Pathol 26(4):359-364.https://doi.org/10.1293/tox.2013-0025
Al-Baqami NM, Hamza RZ (2021) Protective effect of resveratrol against hepatotoxicity of cadmium in male rats: Antioxidant and histopathological approaches. Coatings 11(5):594. https://doi.org/10.3390/coatings11050594
Hartwig A, Asmuss M, Ehleben I et al (2002) Interference by Toxic Metal Ions with DNA Repair Processes and Cell Cycle Control : Molecular Mechanisms 5(Suppl 5):797–799. https://doi.org/10.1289/ehp.02110s5797
Hakansson A, Molin G (2011) Gut Microbiota and Inflammation.Nutrients 3(6):637–682. https://doi.org/10.3390/nu3060637
Sharma S, Singh RL, Kakkar P (2011) Modulation of Bax / Bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem Toxicol 49:770–779. https://doi.org/10.1016/j.fct.2010.11.041
Bao R, Zheng S, Wang X (2017) Selenium protects against cadmium-induced kidney apoptosis in chickens by activating the PI3K / AKT / Bcl-2 signaling pathway. Environ Sci Pollut Res Int 24(25):24:20342–20353. https://doi.org/10.1007/s11356-017-9422-6
Le NV, Guglielmetti S, Arioli S et al (2018) Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy : A Strategy to Promote Immunosurveillance against Lung Metastases Article Modulation of Pulmonary Microbiota by Antibiotic or Probiotic Aerosol Therapy: A Strategy to Promote. Cell Rep 24:3528–3538. https://doi.org/10.1016/j.celrep.2018.08.090
Access O, Karoui-kharrat D, Kaddour H et al (2017) Response of antioxidant enzymes to cadmium- induced cytotoxicity in rat cerebellar granule neurons. Int J Mol Med 14(1):87–92. https://doi.org/10.1515/biol-2017-0013
Trbojevi IS, ÐZ (2010) Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes : Protective role of coenzyme Q 10 and Vitamin E.Reprod. Toxicol 29(2):191–197. https://doi.org/10.1016/j.reprotox.2009.11.009
Zhuang J, Nie G, Yang F et al (2019) Cadmium induces cytotoxicity through oxidative stress-mediated apoptosis pathway in duck renal tubular epithelial cells. Toxicol Vitr 61:104625. https://doi.org/10.1016/j.tiv.2019.104625
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dashtbanei, S., Keshtmand, Z. A Mixture of Multi-Strain Probiotics (Lactobacillus Rhamnosus, Lactobacillus Helveticus, and Lactobacillus Casei) had Anti-Inflammatory, Anti-Apoptotic, and Anti-Oxidative Effects in Oxidative Injuries Induced By Cadmium in Small Intestine and Lung. Probiotics & Antimicro. Prot. 15, 226–238 (2023). https://doi.org/10.1007/s12602-022-09946-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09946-0