Abstract
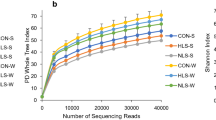
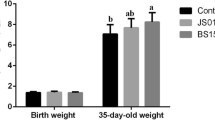
Supplementing suckling piglets with Lactobacillus reuteri isolated from a homologous source improves L. reuteri colonization number in the gastrointestinal tract, which can have health benefits. This study investigated dietary L. reuteri supplementation on the growth and health—including immune status—of piglets, as well as its colonization. A total of 60 sows with similar parity and body weight were allocated into one of three groups after secretion (n = 20 each, with 10 neonatal piglets of each): untreated control, L. reuteri supplementation, and antibiotic treatment. The experimental duration was 28 days, from birth of piglets to their group transferred. For the first 7 days after birth, all neonatal piglets were fed by sows. Piglets in the L. reuteri supplementation group were administered with 1.0 ml L. reuteri fermentation broth containing 5.0 × 107 CFU. From 7 to 28 days, piglets were given basal feed (control), basal feed supplemented with L. reuteri (1.0 × 107 CFU/g), or aureomycin (150 mg/kg). L. reuteri colonization in the distal jejunum and ileum was increased in piglets in the L. reuteri-supplemented as compared to the control group after 28 days, as determined by fluorescence in situ hybridization and real-time PCR analysis. Total Lactobacillus and Bifidobacterium counts in the cecum were higher whereas total aerobic bacteria (Escherichia coli and Staphylococcus) counts were lower in the L. reuteri as compared to the control group. L. reuteri supplementation also improved body antioxidant status and immune function relative to control animals. Strain-specific L. reuteri administered to piglets colonizes the intestinal mucosa and improves cecal microbiota profile and whole-body antioxidant and immune status, leading to better growth and lower morbidity and mortality rates.






Similar content being viewed by others
References
Niu Q, Li P, Hao S, Zhang Y, Kim SW, Li H, Ma X, Gao S, He L, Wu W, Huang X (2015) Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci Rep 5:9938
Yang JJ, Qian K, Wu D, Zhang W, Wu YJ, Xu YY (2017) Effects of different proportions of two bacillus strains on the growth performance, small intestinal morphology, caecal microbiota and plasma biochemical profile of Chinese Huainan partridge shank chickens. J Integr Agric 16:1383–1392
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The international scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Rycroft CE, Jones RM, Gibson GR, Rastall RA (2001) A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microbiol 91:878–887
Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG (2007) Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci U S A 104:7617–7621
Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132:562–575
Kamada N, Chen GY, Inohara N, Nunez G (2013) Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14:685–690
Louis P, Scott KP, Duncan SH, Flint HJ (2007) Understanding the effects of diet on bacterial metabolism in the large intestine. J Appl Microbiol 102:1197–1208
Hooper LV, Macpherson AJ (2010) Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol 10:159–169
Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J (2017) Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol 58:1–14
Wells JM, Rossi O, Meijerink M, van Baarlen P (2011) Epithelial crosstalk at the microbiota–mucosal interface. Proc Natl Acad Sci U S A 108:4607–4614
Sattler VA, Mohnl M, Klose V (2014) Development of a strain-specific real-time PCR assay for enumeration of a probiotic Lactobacillus reuteri in chicken feed and intestine. PLoS One 9:e90208
Rinttila T, Kassinen A, Malinen E, Krogius L, Palva A (2004) Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol 7:1166–1177
De Martinis EC, Duvall RE, Hitchins AD (2007) Real-time PCR detection of 16S rRNA genes speeds most-probable-number enumeration of foodborne listeria monocytogenes. J Food Prot 70:1650–1655
Tajima K, Aminov RI, Nagamine T, Matsui H, Nakamura M, Benno Y (2001) Diet dependent shifts in the bacterial population of the rumen revealed with real-time PCR. Appl Environ Microbiol 67:2766–2774
Yang JJ, Qian K, Wang CL, Wu YJ (2018) Roles of probiotic Lactobacilli inclusion in helping piglets establish healthy intestinal inter-environment for pathogen defense. Probiotics Antimicrob Proteins 2:243–250
Brown RF, Jugg BJ, Harbanm FM, Ashley Z, Kenward CE, Platt J, Hill A, Rice P, Watkins PE (2002) Pathophysiological responses following phosgene exposure in the anaesthetized pig. J Appl Toxicol 22:263–269
Jensen H, Roos S, Jonsson H, Rud I, Grimmer S, van Pijkeren JP, Britton RA, Axelsson L (2014) Role of Lactobacillus reuteri cell and mucus-binding protein a (CmbA) in adhesion to intestinal epithelial cells and mucus in vitro. Microbiology 160:671–681
Meijerink M, Mercenier A, Wells JM (2013) Challenges in translational research on probiotic lactobacilli: from in vitro assays to clinical trials. Benefic Microbes 4:83–100
NRC (National Research Council) (1994) Nutrient Requirement of Poultry, 9th edn. National Academy Press, USA
Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, Fegeros K (2010) Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci 89:58–67
Yang JJ, Xu YY, Qian K, Zhang W, Wu D, Wang CL (2016) Effects of chromium-enriched Bacillus subtilis KT260179 supplementation on growth performance, caecal microbiology, tissue chromium level, insulin receptor expression and plasma biochemical profile of mice under heat stress. Br J Nutr 115:774–781
Donaldson GP, Lee SM, Mazmanian SK (2016) Gut biogeography of the bacterial 579 microbiota. Nat Rev Microbiol 14:20–32
Arora N, Sadovsky Y, Dermody TS, Coyne CB (2017) Microbial vertical transmission during human pregnancy. Cell Host Microbe 21:561–567
Su Y, Chen X, Liu M, Guo X (2017) Effect of three lactobacilli with strain-specific activities on the growth performance, faecal microbiota and ileum mucosa proteomics of piglets. J Anim Sci Biotechnol 8:52
Hou C, Zeng X, Yang F, Liu H, Qiao S (2015) Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Anim Sci Biotechnol 6:14
Rao SC, Athalye-Jape GK, Deshpande GC, Simmer KN, Patole SK (2016) Probiotic supplementation and late-onset Sepsis in preterm infants: a meta-analysis. Pediatrics 137:e20153684
Rheinallt MJ (2016) The influence of the gut microbiota on host physiology: in pursuit of mechanisms. Yale J Biol Med 89:285–297
Zheng X, Duan Y, Dong H, Zhang J (2018) Effects of dietary Lactobacillus plantarum on growth performance, digestive enzymes and gut morphology of Litopenaeus vannamei. Probiotics Antimicrob Proteins 10:504–510
Li H, Lei Z, Chen L, Qi Z, Wang W, Qiao J (2016) Lactobacillus acidophilus, alleviates the inflammatory response to enterotoxigenic Escherichia coli, k88 via inhibition of the nf-κb and p38 mitogen-activated protein kinase signaling pathways in piglets. BMC Microbiol 16:273
Wang H, Ni X, Liu L, Zeng D, Lai J, Qing X, Li G, Pan K, Jing B (2017) Controlling of growth performance, lipid deposits and fatty acid composition of chicken meat through a probiotic, Lactobacillus johnsonii during subclinical Clostridium perfringens infection. Lipids Health Dis 16:38
Harper AF, Kornegay ET, Bryant KL, Thomas HR (1983) Efficacy of virginiamycin and a commercially-available Lactobacillus probiotic in swine diets. Anim Feed Sci Technol 8:69–76
Lei K, Li YL, Yu DY, Rajput IR, Li WF (2013) Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult Sci 92:2389–2395
Yang JJ, Qian K, Zhang W, Xu YY, Wu YJ (2016) Effects of chromium-enriched bacillus subtilis KT260179 supplementation on chicken growth performance, plasma lipid parameters, tissue chromium levels, cecal bacterial composition and breast meat quality. Lipids Health Dis 15:188
Rozs M, Manczinger L, Vágvölgyi C, Kevei F (2001) Secretion of a trypsin-like thiol protease by a new keratinolytic strain of Bacillus licheniformis. FEMS Microbiol Lett 205:221–224
Spanhaak S, Havenaar R, Schaafsma G (1998) The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur J Clin Nutr 52:899–907
Lee YK, Ho PS, Low CS, Arvilommi H, Salminen S (2004) Permanent colonization by Lactobacillus casei is hindered by the low rate of cell division in mouse gut. Appl Environ Microbiol 70:670–674
Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh CS, Colonna M (2017) Lactobacillus reuteri induces gut intraepithelial CD4(+) CD8αα(+) T cells. Science 357:806–810
Lopez P, Gonzalez-Rodriguez I, Sanchez B, Ruas-Madiedo P, Suarez A, Margolles A, Gueimonde M (2012) Interaction of Bifidobacterium bifidum LMG13195 with HT29 cells influences regulatory-T cell associated chemokine receptor expression. Appl Environ Microbiol 78:850–2857
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157:121–141
Engin KN, Yemisci B, Yigit U, Agachan A, Coskun C (2010) Variability of serum oxidative stress biomarkers relative to biochemical data and clinical parameters of glaucoma patients. Mol Vis 16:1260–1271
Ogawa F, Shimizu K, Muroi E, Hara T, Sato S (2011) Increasing levels of serum antioxidant status, total antioxidant power, in systemic sclerosis. Clin Rheumatol 30:921–925
Liu H, Zhang J, Zhang S, Yang F, Thacker PA, Zhang G, Qiao S, Ma X (2014) Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J Agric Food Chem 62:860–866
Munoz-Tamayo R, Laroche B, Walter E, Doré J, Duncan SH, Flint HJ, Leclerc M (2011) Kinetic modeling of lactate utilization and butyrate production by key human colonic bacterial species. FEMS Microbiol Ecol 76:615–624
Funding
The work was sponsored by the fund of Anhui Academy of Agricultural Sciences Key Laboratory Project (No.:18S0404), natural science foundation of Anhui province (No. 1708085QC72), State Key Laboratory of Animal Nutrition (No. 2004D125184f1703), and Anhui Science and Technology Key Project (No. 17030701008) and Key Laboratory of Safety.
Author information
Authors and Affiliations
Contributions
JY designed the study, isolated and cultured L. reuteri yjj, fed the piglets and recorded the growth data, wrote the paper, established the qRT-PCR assay; LL and CW measured the mRNA levels, and HZ was involved in technical direction. CW and MZ had primary responsibility for the final content.
Corresponding author
Ethics declarations
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The experimental guidelines, the treatment, housing, and husbandry conditions conformed to Institutional Animal Care and Use Committee of China. The experimental protocols in this study including animal husbandry and slaughter were approved by the Institution of Animal Science and Welfare of Anhui Province (no. IASWAP20170685).
Consent for Publication
All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Wang, C., Liu, L. et al. Lactobacillus reuteri KT260178 Supplementation Reduced Morbidity of Piglets Through Its Targeted Colonization, Improvement of Cecal Microbiota Profile, and Immune Functions. Probiotics & Antimicro. Prot. 12, 194–203 (2020). https://doi.org/10.1007/s12602-019-9514-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-9514-3