Abstract
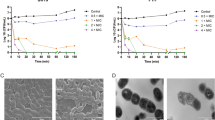
Salmonella is an important zoonotic pathogen and is a major cause of gastrointestinal diseases worldwide. The current serious problem of antibiotic abuse has prompted the search for new substitutes for antibiotics. JH-3 is a small antimicrobial peptide with broad-spectrum bactericidal activity. In this study, we showed that JH-3 has good bactericidal activity towards the clinical isolate Salmonella enterica serovar Typhimurium strain CVCC541. The minimum inhibitory concentration (MIC) of JH-3 against this bacterium was determined to be 100 μg/mL, which could decrease the number of CVCC541 cells by 1000-fold in vitro within 5 h. The transmission electron microscopy (TEM) results showed that JH-3 can damage the cell wall and membrane of CVCC541, leading to the leakage of cell contents and subsequent cell death. To measure the bactericidal activity of CVCC541-infected mice were treated intraperitoneally 40 or 10 mg/kg JH-3 at 2 h or 3 days postinfection. Our results showed that treatment with 40 mg/kg JH-3 at 2 h postinfection had the best therapeutic effect and could significantly protect mice from a lethal dose of CVCC541. Furthermore, the clinical symptoms, bacterial burden in blood and organs, and intestinal pathological changes were all decreased and were close to normal. This study examined the therapeutic effect of the antimicrobial peptide JH-3 against S. enterica CVCC541 infection for the first time and determined the therapeutic effect of different JH-3 doses and treatment times, laying the foundation for studies of new antimicrobial agents.







Similar content being viewed by others
References
Owen KA, Meyer CB, Bouton AH, Casanova JE (2014) Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog 10(6):e1004159. https://doi.org/10.1371/journal.ppat.1004159
Crump JA, Luby SP, Mintz ED (2004) The global burden of typhoid fever. Bull World Health Organ 82(5):346–353. https://doi.org/10.1590/S0042-96862004000500008
Kozak GK, MacDonald D, Landry L, Farber JM (2013) Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J Food Prot 76(1):173–183. https://doi.org/10.4315/0362-028X.JFP-12-126
Fabrega A, Vila J (2013) Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin Microbiol Rev 26(2):308–341. https://doi.org/10.1128/CMR.00066-12
Amajoud N, Bouchrif B, Maadoudi ME, Senhaji NS, Karraouan B, Harsal AE, Abrini JE (2017) Prevalence, serotype distribution, and antimicrobial resistance of Salmonella isolated from food products in Morocco. J Infect Dev Ctries 11(2):136–142. https://doi.org/10.3855/jidc.8026
Fernandes SA, Camargo CH, Francisco GR, Bueno MFC, Garcia DO, Doi Y, Tiba Casas MR (2017) Prevalence of extended-spectrum beta-lactamases CTX-M-8 and CTX-M-2-producing Salmonella serotypes from clinical and nonhuman isolates in Brazil. Microb Drug Resist 23(5):580–589. https://doi.org/10.1089/mdr.2016.0085
Iwamoto M, Reynolds J, Karp BE, Tate H, Fedorka-Cray PJ, Plumblee JR, Hoekstra RM, Whichard JM, Mahon BE (2017) Ceftriaxone-resistant nontyphoidal Salmonella from humans, retail meats, and food animals in the United States, 1996-2013. Foodborne Pathog Dis 14(2):74–83. https://doi.org/10.1089/fpd.2016.2180
Correia S, Nunes-Miranda JD, Pinto L, Santos HM, de Toro M, Saenz Y, Torres C, Capelo JL, Poeta P, Igrejas G (2014) Complete proteome of a quinolone-resistant Salmonella Typhimurium phage type DT104B clinical strain. Int J Mol Sci 15(8):14191–14219. https://doi.org/10.3390/ijms150814191
Brown KL, Poon GFT, Birkenhead D, Pena OM, Falsafi R, Dahlgren C, Karlsson A, Bylund J, Hancock REW, Johnson P (2011) Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J Immunol 186(9):5497–5505. https://doi.org/10.4049/jimmunol.1002508
Zhang M, Volpert O, Shi YH, Bouck N (2000) Maspin is an angiogenesis inhibitor. Nat Med 6(2):196–199. https://doi.org/10.1038/72303
Hu J, Xu M, Hang B, Wang L, Wang Q, Chen J, Song T, Fu D, Wang Z, Wang S, Liu X (2011) Isolation and characterization of an antimicrobial peptide from bovine hemoglobin α-subunit. World J Microbiol Biotechnol 27(4):767–771. https://doi.org/10.1007/s11274-010-0514-4
Zhang Q, Xu Y, Wang Q, Hang B, Sun Y, Wei X, Hu J (2015) Potential of novel antimicrobial peptide P3 from bovine erythrocytes and its analogs to disrupt bacterial membranes in vitro and display activity against drug-resistant bacteria in a mouse model. Antimicrob Agents Chemother 59(5):2835–2841. https://doi.org/10.1128/AAC.04932-14
Pereira V, Lopes C, Castro A, Silva J, Gibbs R, Teixeira P (2009) Characterization for enterotoxin production, virulence factors, and antibiotic susceptibility of Staphylococcus aureus isolates from various foods in Portugal. Food Microbiol 26(3):278–282. https://doi.org/10.1016/j.fm.2008.12.008
Jones A, Georg M, Maudsdotter L, Jonsson A (2009) Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J Bacteriol 191(12):3861–3868. https://doi.org/10.1128/JB.01313-08
Wang L, Qin W, Zhai R, Liu S, Zhang H, Sun C, Feng X, Gu J, Du C, Han W, Langford PR, Lei L (2015) Differential gene expression profiling of Actinobacillus pleuropneumoniae during induction of primary alveolar macrophage apoptosis in piglets. Microb Pathog 78:74–86. https://doi.org/10.1016/j.micpath.2014.11.017
Wang L, Zhao X, Zhu C, Xia X, Qin W, Li M, Wang T, Chen S, Xu Y, Hang B, Sun Y, Jiang J, Richard LP, Lei L, Zhang G, Hu J (2017) Thymol kills bacteria, reduces biofilm formation, and protects mice against a fatal infection of Actinobacillus pleuropneumoniae strain L20. Vet Microbiol 203:202–210. https://doi.org/10.1016/j.vetmic.2017.02.021
Martz SE, McDonald JAK, Sun J, Zhang Y, Gloor GB, Noordhof C, He S, Gerbaba TK, Blennerhassett M, Hurlbut DJ, Allen-Vercoe E, Claud EC, Petrof EO (2015) Administration of defined microbiota is protective in a murine Salmonella infection model. Sci Rep 5(16094). https://doi.org/10.1038/srep16094
Wang L, Qin W, Zhang J, Bao C, Zhang H, Che Y, Sun C, Gu J, Feng X, Du C, Han W, Richard PL, Lei L (2016) Adh enhances Actinobacillus pleuropneumoniae pathogenicity by binding to OR5M11 and activating p38 which induces apoptosis of PAMs and IL-8 release. Sci Rep 6(24058). https://doi.org/10.1038/srep24058
Yang F, Ma Q, Lei L, Huang J, Ji Q, Zhai R, Wang L, Wang Y, Li L, Sun C, Feng X, Han W (2014) Specific humoral immune response induced by propionibacterium acnes can prevent Actinobacillus pleuropneumoniae infection in mice. Clin Vaccine Immunol 21(3):407–416. https://doi.org/10.1128/CVI.00667-13
Wang L, Qin W, Yang S, Zhai R, Zhou L, Sun C, Pan F, Ji Q, Wang Y, Gu J, Feng X, Du C, Han W, Langford PR, Lei L (2015) The Adh adhesin domain is required for trimeric autotransporter Apa1-mediated Actinobacillus pleuropneumoniae adhesion, autoaggregation, biofilm formation and pathogenicity. Vet Microbiol 177(1–2):175–183. https://doi.org/10.1016/j.vetmic.2015.02.026
Schwan WR, Huang XZ, Hu L, Kopecko DJ (2000) Differential bacterial survival, replication, and apoptosis-inducing ability of Salmonella serovars within human and murine macrophages. Infect Immun 68(3):1005–1013. https://doi.org/10.1128/IAI.68.3.1005-1013.2000
Herikstad H, Motarjemi Y, Tauxe RV (2002) Salmonella surveillance: a global survey of public health serotyping. Epidemiol Infect 129(1):1–8. https://doi.org/10.1017/epidmiolinfect.S0950268802006842
Penesyan A, Gillings M, Paulsen IT (2015) Antibiotic discovery: combatting bacterial resistance in cells and in biofilm communities. Molecules 20(4):5286–5298. https://doi.org/10.3390/molecules.20045286
Benincasa M, Pelillo C, Zorzet S, Garrovo C, Biffi S, Gennaro R, Scocchi M (2010) The proline-rich peptide Bac7(1-35) reduces mortality from Salmonella Typhimurium in a mouse model of infection. BMC Microbiol 10:178. https://doi.org/10.1186/1471-2180-10-178
Yoo JH, Ho S, Tran DH, Cheng M, Bakirtzi K, Kukota Y, Ichikawa R, Su B, Tran DH, Hing TC, Chang I, Shih DQ, Issacson RE, Gallo RL, Fiocchi C, Pothoulakis C, Koon HW (2015) Anti-fibrogenic effects of the anti-microbial peptide cathelicidin in murine colitis-associated fibrosis. Cell Mol Gastroenterol Hepatol 1(1):55–74.e1. https://doi.org/10.1016/j.jcmgh.2014.08.001
Dong N, Ma Q, Shan A, Lv Y, Hu W, Gu Y, Li Y (2012) Strand length-dependent antimicrobial activity and membrane-active mechanism of arginine- and valine-rich β-hairpin-like antimicrobial peptides. Antimicrob Agents Chemother 56(6):2994–3003. https://doi.org/10.1128/AAC.06327-11
Wu X, Wang Z, Li X, Fan Y, He G, Wan Y, Yu C, Tang J, Li M, Zhang X, Zhang H, Xiang R, Pan Y, Liu Y, Lu L, Yang L (2014) In vitro and in vivo activities of antimicrobial peptides developed using an amino acid-based activity prediction method. Antimicrob Agents Chemother 58(9):5342–5349. https://doi.org/10.1128/AAC.02823-14
Chen H, Su P, Chang Y, Wu S, Liao Y, Yu H, Lauderdale T, Chang K, Shih C (2013) Identification of a novel antimicrobial peptide from human hepatitis B virus core protein arginine-rich domain (ARD). PLoS Pathog 9(6):e1003425. https://doi.org/10.1371/journal.ppat.1003425
Lee E, Kim J, Jeon D, Jeong K, Shin A, Kim Y (2015) Functional roles of aromatic residues and helices of papiliocin in its antimicrobial and anti-inflammatory activities. Sci Rep 5:12048. https://doi.org/10.1038/srep12048
Lv Y, Wang J, Gao H, Wang Z, Dong N, Ma Q, Shan A (2014) Antimicrobial properties and membrane-active mechanism of a potential alpha-helical antimicrobial derived from cathelicidin PMAP-36. PLoS One 9(1):e86364. https://doi.org/10.1371/journal.pone.0086364
Xu W, Zhu X, Tan T, Li W, Shan A (2014) Design of embedded-hybrid antimicrobial peptides with enhanced cell selectivity and anti-biofilm activity. PLoS One 9(6):e98935. https://doi.org/10.1371/journal.pone.0098935
Hing TC, Ho S, Shih DQ, Ichikawa R, Cheng M, Chen J, Chen X, Law I, Najarian R, Kelly CP, Gallo RL, Targan SR, Pothoulakis C, Koon HW (2013) The antimicrobial peptide cathelicidin modulates Clostridium difficile-associated colitis and toxin A-mediated enteritis in mice. Gut 62(9):1295–1305. https://doi.org/10.1136/gutjnl-2012-302180
Kang JK, Hwang JS, Nam HJ, Ahn KJ, Seok H, Kim S, Yun EY, Pothoulakis C, Lamont JT, Kim H (2011) The insect peptide coprisin prevents Clostridium difficile-mediated acute inflammation and mucosal damage through selective antimicrobial activity. Antimicrob Agents Chemother 55(10):4850–4857. https://doi.org/10.1128/AAC.00177-11
Kim DH, Hwang JS, Lee IH, Nam ST, Hong J, Zhang P, Lu LF, Lee J, Seok H, Pothoulakis C, Lamont JT, Kim H (2016) The insect peptide CopA3 increases colonic epithelial cell proliferation and mucosal barrier function to prevent inflammatory responses in the gut. J Biol Chem 291(7):3209–3223. https://doi.org/10.1074/jbc.M115.682856
Czyzewski AM, Jenssen H, Fjell CD, Waldbrook M, Chongsiriwatana NP, Yuen E, Hancock REW, Barron AE (2016) In vivo, in vitro, and in silico characterization of peptoids as antimicrobial agents. PLoS One 11(2):e0135961. https://doi.org/10.1371/journal.pone.0135961
Schlusselhuber M, Torelli R, Martini C, Leippe M, Cattoir V, Leclercq R, Laugier C, Groetzinger J, Sanguinetti M, Cauchard J (2013) The equine antimicrobial peptide eCATH1 is effective against the facultative intracellular pathogen Rhodococcus equi in mice. Antimicrob Agents Chemother 57(10):4615–4621. https://doi.org/10.1128/AAC.02044-12
Papareddy P, Kalle M, Bhongir RKV, Moergelin M, Malmsten M, Schmidtchen A (2014) Antimicrobial effects of helix D-derived peptides of human antithrombin III. J Biol Chem 289(43):29790–29800. https://doi.org/10.1074/jbc.M114.570465
Acknowledgments
Lei Wang and Xueqin Zhao contributed equally to this work. We thank Xiaojing Xia, Chunling Zhu, Wanhai Qin and Hanna Fotina for providing language assistance. We thank Yanzhao Xu, Bolin Hang, Yawei Sun, Shijun Chen, Huihui Zhang and Jinqing Jiang for writing and editing assistance. We also thank Jianhe Hu and Gaiping Zhang for guidance and for help designing the article.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 31672559, 31702259 and 31372447), the National Key Research and Development Program of China (2016YFD0500708-04), the Program for Science Technology Innovation Talents in Universities of Henan Province (14HASTIT026), and the Excellent Youth Foundation of Henan Scientific Committee (174100510005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Female BALB/c mice (6 to 8 weeks old, body weight of 18 to 22 g) were purchased from the Animal Center of Zhengzhou University (No. 41003100003648). All animal studies were conducted according to the experimental practices and standards of the Animal Welfare and Research Ethics Committee at Zhengzhou University. The study was approved by the Animal Centre of Zhengzhou University.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Zhao, X., Xia, X. et al. Antimicrobial Peptide JH-3 Effectively Kills Salmonella enterica Serovar Typhimurium Strain CVCC541 and Reduces Its Pathogenicity in Mice. Probiotics & Antimicro. Prot. 11, 1379–1390 (2019). https://doi.org/10.1007/s12602-019-09533-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-019-09533-w