Abstract
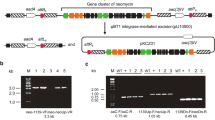
Nisin is a small antimicrobial peptide produced by several subset strains of Lactococcus lactis. To improve nisin yield in the producer L. lactis LS01, we proposed a successive fusion of nisA with nisRK and nisFEG into a single shuttle expression vector pMG36e under the control of the native strong constitutive promoter p32. Subsequently, the recombinant vectors were transplanted into the producer cell through electroporation. Nisin productivity was determined through sodium dodecyl sulfate-polyacrylamide gel electrophoresis and bioactivity assays. Expression of nisin peptide was detected by agar diffusion bioassay, and the transcriptional levels of the target genes involved in nisin biosynthesis were investigated via semi-quantitative reverse transcription PCR expression analysis using 16S ribosomal RNA (rRNA) as an internal control. Results suggested that the introduction of empty plasmid did not affect nisin production of L. lactis LS01, whereas by our rational construction and screening, the engineered strain co-overexpressing nisA, nisRK, and nisFEG achieved a maximum increment in bioactive nisin production with a yield of 2470 IU/ml in shake flasks and 4857 IU/ml in 1.0-l fermenters, which increased by approximately 66.3 and 52.6% (P < 0.05), respectively, compared with that of the original strain under the given fermentation conditions. Meanwhile, the transcriptional analysis revealed that the expression of most of these multicopy genes except nisE at transcriptional level were upregulated in the two recombinant strains (LS01/pAR and LS01/pARF), possibly contributing to the improved nisin production. Therefore, this study would provide a potential strategy to improve the economic benefits of nisin manufacture for large-scale industrial production.




Similar content being viewed by others
References
Cheigh CI, Pyun YR (2005) Nisin biosynthesis and its properties. Biotechnol Lett 27(21):1641–1648
Hurst A (1981) Nisin. Adv Appl Microbiol 27:85–123
Hampikyan H (2009) Efficacy of nisin against Staphylococcus aureus in experimentally contaminated sucuk, a Turkish-type fermented sausage. J Food Prot 72:1739–1743
Periago PM, Moezelaar R (2001) Combined effect of nisin and carvacrol at different pH and temperature levels on the viability of different strains of Bacillus cereus. Int J Food Microbiol 68(1):141–148
Severina E, Severin A, Tomasz A (1998) Antibacterial efficacy of nisin against multidrug-resistant gram-positive pathogens. J Antimicrob Chemother 41:341–347. doi:10.1093/jac/41.3.341
Delves-Broughton J, Blackburn P, Evans R, Hugenholtz J (1996) Applications of the bacteriocin, nisin. Antonie Van Leeuwenhoek 69(2):193–202. doi:10.1007/BF00399424
Food and Drug Administration (1988) Nisin preparation: affirmation of GRAS status as direct human food ingredient. Fed Regist 53(66):11247–11313
De Kwaadsteniet M, Doeschate KT, Dicks LMT (2009) Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett Appl Microbiol 48(1):65–70. doi:10.1111/j.1472-765X.2008.02488.x
Gordon YJ, Romanowski EG, McDermott AM (2005) A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30(7):505–515
Sheldon BW, Schuman JD (1998) Thermal and biological treatments to control psychrotrophic pathogens. Poult Sci 75:1126–1132
Tong Z, Ni L, Ling J (2014) Antibacterial peptide nisin: a potential role in the inhibition of oral pathogenic bacteria. Peptides 60:32–40
Aranha C, Gupta S, Reddy KVR (2004) Contraceptive efficacy of antimicrobial peptide nisin: in vitro and in vivo studies. Contraception 69(4):333–338
Kuipers OP, Beerthuyzen MM, de Ruyter PGGA, Luesink EJ, de Vos WM (1995) Autoregulation of nisin biosynthesis in Lactococcus lactis by signal transduction. J Biol Chem 270:27299–27304. doi:10.1074/jbc.270.45.27299
Kleerebezem M, Quadri LE (2001) Peptide pheromone-dependent regulation of antimicrobial peptide production in gram-positive bacteria: a case of multicellular behavior. Peptides 22:1579–1596
Siegers K, Heinzmann S, Entian KD (1996) Biosynthesis of lantibiotic nisin. Post-translational modification of its prepeptide occurs at a multimeric membrane-associated lanthionine synthetase complex. J Biol Chem 271:12294–12301. doi:10.1074/jbc.271.21.12294
Cheigh CI, Park H, Choi HJ, Pyun YR (2005) Enhanced nisin production by increasing genes involved in nisin Z biosynthesis in Lactococcus lactis subsp. lactis A164. Biotechnol Lett 27:155–160. doi:10.1007/s10529-004-7661-3
De Ruyter PG, Kuipers OP, Beerthuyzen MM, van Alen-Boerrigter I, De Vos WM (1996) Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J Bacteriol 178(12):3434–3439. doi:10.1128/jb.178.12.3434-3439.1996
Kleerebezem M, Quadri LEN, Kuipers OP, de Vos WM (1997) Quorum-sensing by peptide pheromones and two-component signal-transduction systems in gram positive bacteria. Mol Microbiol 24:895–904. doi:10.1046/j.1365-2958.1997.4251782.x
Kleerebezem M, De Vos WM, Kuipers OP (1998) The lantibiotics nisin and subtilin act as extracellular regulators of their own biosynthesis. In: Dunny GM, Winams SC (eds) Cell–cell signalling in bacteria. American Society for Microbiology, Washington, D.C.
Ra R, Qiao M, Immonen T, Pujana I, Saris PEJ (1996) Genes responsible for nisin synthesis, regulation and immunity form a regulon of two operons and are induced by nisin in Lactococcus lactis N8. Microbiology 142:1282–1288. doi:10.1099/13500872-142-5-1281
Lubelski J, Rink R, Khusainov R, Moll G, Kuipers O (2008) Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin. Cell Mol Life Sci 65(3):455–476. doi:10.1007/s00018-007-7171-2
Kim WS, Hall RJ, Dunn NW (1998) Improving nisin production by increasing nisin immunity/resistance genes in the producer organism Lactococcus lactis. Appl Microbiol Biotechnol 50:429–433. doi:10.1007/s002530051316
Kong W, Lu T (2014) Cloning and optimization of a nisin biosynthesis pathway for bacteriocin harvest. ACS Synth Biol 3:439–445. doi:10.1021/sb500225r
Haina Li (2011) Screening of high yield nisin-producing strains and optimization of its fermentation conditions. Dissertation. Shandong University
O'sullivan DJ, Klaenhammer TR (1993) Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol 59(8):2730–2733
Gerber SD, Solioz M (2007) Efficient transformation of Lactococcus lactis IL1403 and generation of knock-out mutants by homologous recombination. J Basic Microbiol 47(3):281–286. doi:10.1002/jobm.200610297
Papagianni M, Avramidis N, Filioussis G (2007) High efficiency electro-transformation of Lactococcus lactis spp. lactis cells pretreated with lithium acetate and dithiothreitol. BMC Biotechnol 7(1):1. doi:10.1186/1472-6750-7-15
Daba H, Pandian S, Gosselin JF, Simard RE, Huang J, Lacroix C (1991) Detection and activity of a bacteriocin by Leuconostoc mesenteroides. Appl Environ Microbiol 57:3450–3455
Marone M, Mozzetti S, De Ritis D, Pierelli L, Scambia G (2001) Semi-quantitative RT-PCR analysis to assess the expression levels of multiple transcripts from the same sample. Biol Proced Online 3(1):19–25
Yadav P, Mukesh M, Kataria RS, Yadav A, Mohanty AK (2012) Semi-quantitative RT-PCR analysis of fat metabolism genes in mammary tissue of lactating and non-lactating water buffalo (Bubalus bubalis). Trop Anim Health Prod 44(4):693–696. doi:10.1007/s11250-011-9988-9
Cheigh CI, Choi HJ, Park H, Kim SB, Kook MC, Kim TS, Hwang JK, Pyun YR (2002) Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. J Biotechnol 95:225–235
De Vuyst L, Vandamme EJ (1992) Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. Microbiology 138(3):571–578
Zamfir M, Callewaert R, Cornea PC, De Vuyst L (2000) Production kinetics of acidophilin 801, a bacteriocin produced by Lactobacillus acidophilus IBB801. FEMS Microbiol Lett 190:305–308
Kim WS, Hall RJ, Dunn NW (1997) The effect of nisin concentration and nutrient depletion on nisin production of Lactococcus lactis. Appl Microbiol Biotechnol 48(4):449–453
Tafreshi SH, Mirdamadi S, Norouzian D et al (2010) Effect of non-nutritional factors on nisin production. Afr J Biotechnol 9(9):1382–1391
Acknowledgements
This work was financially supported by the Shandong Province High-tech Industry Major Projects (No. 2015ZDJS04002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ni, ZJ., Zhang, Xy., Liu, F. et al. Effect of Co-overexpression of Nisin Key Genes on Nisin Production Improvement in Lactococcus lactis LS01. Probiotics & Antimicro. Prot. 9, 204–212 (2017). https://doi.org/10.1007/s12602-017-9268-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9268-8