Abstract
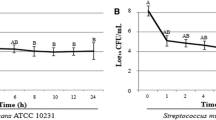
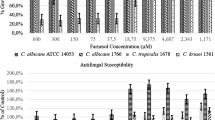
Preparations of probiotic bifidobacterial and lactobacillus lectins possessed system affinity to mannan and mucin-type polymers. It was shown that these lectins possess fungistatic and fungicidal activities against nystatin-resistant Candida albicans clinical strains. Lectins revealed destructive properties with respect to C. albicans and Staphylococcus aureus biofilms, depending on clinical strain origin and lectin preparation type. Synergistic antipathogen activities between lectins and between lectins and nystatin were observed. In the presence of lectins, pathogen biofilm degradation occurred in sequential steps, including biofilm refinement, appearance of edge cavities, segmentation, detachment of fragments and their lysis. Fungal response to lectins was more complex compared to that of staphylococci. Cold stress improved pictures of lectin antipathogen action. The data indicate that probiotic bacterial lectins are members of a new class of antimicrobials—destructors of pathogen biofilms.


Similar content being viewed by others
Abbreviations
- aLL:
-
Acidic lectins of lactobacilli
- aLB:
-
Acidic lectins of bifidobacteria
- bLL:
-
Basic lectins of lactobacilli
- bLB:
-
Basic lectins of bifidobacteria
- RPMI-HEPES:
-
RPMI-1640 plus HEPES pH 7.2–7.4
- PAA(b):
-
Polyacrylamide (biotinylated)
- PBS:
-
Phosphate saline buffer pH 7.2–7.4
- PHA:
-
Phytohaemagglutinin
References
Lakhtin VM, Alyoshkin VA, Lakhtin MV, Afanasyev SS, Pospelova VV, Shenderov BA (2006) Lectins, adhesins and lectin-like substances of lactobacilli and bifidobacteria. Vestnik Rossiiskoy Akademii Meditsinskih Nauk No 1:28–34 (in russian)
Lakhtin VM, Lakhtin MV, Pospelova VV, Shenderov BA (2007) Lectins of lactobacilli and bifidobacteria. II. Probiotic lectins of lactobacilli and bifidobacteria as possible signal molecules regulating inter- and intrapopulation relationships between bacteria and between bacteria and the host. Microb Ecol Health Dis 19:153–157
Lakhtin VM, Lakhtin MV, Afanasyev SS, Alyoshkin VA, Nesvizhsky YV, Pospelova VV (2008) [General properties and functioning principles of lectins in biological systems (in russian). Vestnik Rossiiskoy Akademii Meditsinskih Nauk No 3:37–42
Lakhtin VM, Alyoshkin VA, Lakhtin MV, Afanasyev SS, Pospelova VV (2010) Lectins of symbiotic microorganisms. The overview. Beneficial Microbes. (Submitted for publication)
Lakhtin VM (1987) [Lectins for investigation of proteins and carbohydrates (in russian)]. In: Klyosov A (ed) Itogi Nauki I Tehniki, Seriya Biotehnologiya [Reviews of Science and Technique, Series Biotechnology (in russian)], vol 2, Moscow, Vsesoyuzniy Institut Nautchnoy I Tehnitcheskoy Informatsii [VINITI (in russian)], pp 4–290
Lakhtin VM (1990) Biotechnological aspects of lectins. In: Kocourek J, Freed D (eds) Lectins: Biology, Biochemistry, and Clinical Biochemistry, vol 7. Sigma Chemical Company, St Louis, pp 417–426
Lakhtin VM, Afanasyev SS, Alyoshkin VA, Nesvizhsky YV, Pospelova VV, Lakhtin MV et al (2008) Strategical aspects of constructing probiotics of the future. Vestnik Rossiiskoy Akademii Meditsinskih Nauk No 2:33–44 (in russian)
Lebeer S, Vanderleyden J, De Keersmaecker SCJ (2008) Genes and molecules of lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72:728–764
Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthesy-Theulaz IE (2006) GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with host and the gastric pathogen Helicobacter pylori. Infect Immun 74:425–434
Garotte GL, Delfederico L, Bibiloni R, Abraham AG, Perez PF, Semorile L, De Antoni GL (2004) Lactobacilli isolated from kefir grains: evidence of the presence of S-layer proteins. J Dairy Res 71:222–230
Lakhtin VM, Lakhtin MV, Pospelova VV, Shenderov BA (2006) Lactobacilli and bifidobacteria lectins as possible signal molecules regulating intra- and interpopulation bacteria-bacteria and host-bacteria relationships. Part I. Methods of bacterial lectin isolation, physicochemical characterization and some biological activity investigation. Microb Ecol Health Dis 18:55–60
Lakhtin VM, Afanasyev SS, Alyoshkin VA, Nesvizhsky YV, Lakhtin MV, Shubin VV et al (2009) Classification of lectins as universal regulator molecules of biological systems. Vestnik Rossiiskoy Akademii Meditsinskih Nauk No 3:36–43 (in russian)
Matsumura A, Saito T, Arakuni M, Kitazawa H, Kawai Y, Itoh T (1999) New binding assay and preparative trial of cell-surface lectin from Lactobacillus acidophilus group lactic acid bacteria. J Dairy Sci 82:2525–2529
Pretzer G, Snel J, Molenaar D, Wiersma A, Bron PA, Lambert J, de Vos WM, Vandermeer R, Smits M, Kleerebezem M (2005) Biodiversity-based identification and functional characterization of the Mannose-specific adhesion of Lactobacillus plantarum. J Bacteriolol 187:6128–6136
Uchida H, Kinoshita H, Kawai Y, Kitazawa H, Miura K, Shiiba K et al (2006) Lactobacilli binding human A-antigen expressed in intestinal mucosa. Res Microbiol 157:659–665
Pospelova VV, Cherepanova YV (2007) Probiotic acilact and fields of its application. In: Proceedings of the IDF international symposium on lactose and its derivatives (May 14–17, 2007, Moscow). Book of Abstracts, Moscow, Russia, p 328
Aleshkin VA, Amerhanova AM, Pospelova VV, Afanasyev SS, Shenderov BA (2008) History, present situation, and prospects of probiotic research conducted in the GN Gabrichevsky Institute for Epidemiology and Microbiology. Microb Ecol Health Dis 20:113–115
Lakhtin MV, Lakhtin VM, Alyoshkin VA, Nesvizhsky YV, Afanasyev SS, Pospelova VV (2010) The role of lectins from probiotic microorganisms in sustaining the macroorganism. Vestnik Rossiiskoy Akademii Meditsinskih Nauk No 2:3–8 (in russian)
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lakhtin MV, Lakhtin VM, Afanasyev SS, Kokhanovskaya NA, Pozhalostina LV, Korsun VF (2009) Therapeutic and probiotic potential of lectin-containing phytopreparations. New approaches using example of phytocomplex of Endocrinol (in russian). Praktitcheskaya Fitoterapiya (Moskva) No 1:5–12
Waddel WJ (1956) A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med 48:311–314
Suslov AP, Golovin VP, Skvortsov VT, Korontsvit TA (1989) Screening test of cell migration from microcultures in vitro. Immunologiya (Moskva) No 2:73–76 (in russian)
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12:39–86
Lakhtin VM (1994) Lectin sorbents in microbiology. In: Doyle RJ, Slifkin M (eds) Lectin-microorganism interactions. Marcel Dekker, New York, pp 249–298
Lakhtin MV, Lakhtin VM, Alyoshkin VA, Afanasyev SS, Korsun VF (2010) Phytolectins and phytoenzymes in biology and medicine. Praktitcheskaya Fitoterapiya (Moskva) No 1:12–18 (in russian)
Perumal P, Mekala S, Chaffin WL (2007) Role for cell density in antifungal drug resistance in Candida albicans biofilms. Antimicrob Agents Chemother 51:2454–2463
LaFleur MD, Kumamoto CA, Lewis K (2006) Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother 50:3839–3846
Otto M (2009) Staphylococcal biofilms. Curr Topics Microbiol Immunol 322:207–228
Stichternoth C, Ernst JF (2009) Hypoxic adaptation by Efg1 regulates biofilm formation by Candida albicans. Appl Environm Microbiol 75:3663–3672
Lakhtin VM, Lakhtin MV, Korsun VF, Shenderov BA (2008) Mutual potential of lectins of probiotic microorganisms and fungi in conditions of organization and functioning of the model eukaryotic cell biofilms—for further use in clinical practice within phytocompositions. Praktitcheskaya Fitoterapiya (Moskva) No 2:11–17 (in russian)
Senevirathe CJ, Jin LJ, Samaranayake YH, Samaranayake LP (2008) Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob Agents Chemother 52:3259–3266
Masuoka J, Hazen KC (2004) Cell wall mannan and cell surface hydrophobicity in Candida albicans serotype A and B strains. Infect Immun 72:6230–6236
Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA (2007) Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infect Immun 75:2612–2620
Acknowledgments
The authors thank Prof. Chikindas and all other reviewers for fruitful discussion and value comments and recommendations. The authors also thank Katia Noll Sutyak for the technical help in preparing this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakhtin, M., Alyoshkin, V., Lakhtin, V. et al. Probiotic Lactobacillus and Bifidobacterial Lectins Against Candida albicans and Staphylococcus aureus Clinical Strains: New Class of the Pathogen Biofilm Destructors. Probiotics & Antimicro. Prot. 2, 186–196 (2010). https://doi.org/10.1007/s12602-010-9046-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-010-9046-3