Abstract
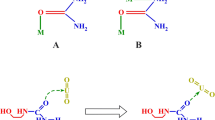
It is very tedious to separate thorium and rare earth elements from their accompanying constituents in low-grade monazite (concentrate 50%) containing large amount of phosphorus species, illiminite, silica and zircon. Therefore, trials have been suggested to develop a new procedure to enhance the separation process of the light lanthanides from low-grade Egyptian monazite concentrates. The first point is focused on the removal of phosphorus species from the digested low-grade monazite with sulfuric acid in order to get more convenient separation. The removal was accomplished by extractive washing of sulfate paste with different alcohols. The results showed that the extractive removal of phosphorus species was not effective due to the complex nature of low-grade monazite concentrate. The second point is focused on the enhancement of separation process of uranium, thorium and rare earth elements by new ratio of sulfuric acid and develops the classical separation process. By this modified procedure, the overall results obtained revealed that the unleached residue contains mainly Si, Fe, Zr, and Ti while the elements of main consideration such as uranium, thorium and light rare earth elements were completely leached. Thorium and light rare earth elements (LREEs) were directly separated as white precipitate while the uranium was moved to the green acid solution with most of phosphorus species. This throws light on the possibility of separation of thorium and light rare earth elements from uranium, which represents a novel method.
Similar content being viewed by others
References
Awwad, N.S., El-Nadi, Y. A., Hamed, M. M. (2013) Successive processes for purification and extraction of phosphoric acid produced by wet process. Chem. Engg. Process., v.74, pp.69–74.
Borai, E. H., Eid, M. A., Aly, H. F.(2002) Determination of REEs distribution in monazite and xenotime minerals by ion chromatography and ICP-AES. Anal. Bioanal. Chem., v.372, pp.537–541.
Borai, E. H., Abd El-Ghany, M.S., Ahmed I. M., Hamed, M. M., Shahr El-Din A.M., Aly, H. F. (2016) Modified acidic leaching for selective separation of thorium, phosphate and rare earth concentrates from Egyptian crude monazite. Internat. Jour. Miner. Process., v.149, pp.34–41.
Brisson, V.L., Zhuang, W.Q., Alvarez-Cohen,.L. (2016) Bioleaching of rare earth elements from monazite sand. Biotechnol. Bioeng., v.113, pp.339–348.
Franken, K. M. and Roast-Leach, A. (1995) A Roast-Leach Process for Extraction of Rare Earths from Complex Monazite-Xenotime Concentrates. Sep. Sci. Tech., v.30, pp.1941–1949.
Gupta, C.K. and Krishnamurthy, N. (2005) Extraction Metallurgy of Rare Earths. Boca Raton, FL: CRC.
Gupta, C.K. and Mukherjee, T.K. (1990) Hydrometallurgy in Extraction Processes Boca Raton, FL: CRC.
Hughes, K.C. and Carswell, D. J. (1970) Separation of the Principal Components in Monazite. Analyst, v.95, pp.302–303.
Ja, Y.G. (1979) Decomposition of Monazite Sand. Jour. Korean Chem. Soc., v.23, pp.136–140.
Kim, E. and Osseo-Asare, K.(2012) Aqueous stability of thorium and rare earth metals in monazite hydrometallurgy: Eh–pH diagrams for the systems Th–, Ce–, La–, Nd–(PO4)–(SO4)–H2O at 25°C. Hydrometallurgy, v.113-114, pp.67–78.
Kumari, A., Panda, A. R., Jha, M. K., Jin, Y. L., Kumarb, J., Kuma, V. (2015) Thermal treatment for the separation of phosphate and recovery of rare earth metals (REMs) from Korean monazite. Jour. Indian Eng. Chem., v.21, pp.696–703.
Moeller, T. (1973) the Chemistry of the Lanthanides: Pergamum Texts in Inorganic Chemistry.
Marczenko, Z. and Balcerzak, M. (2001) Separation, Preconcentration and Spectrophotometry in Inorganic Analysis.
Nascimento, M. R. L., Fatibello-Filho, O., Teixeira, L. A. (2004) Recovery of uranium from acid mine drainage waters by ion exchange. Mineral Processing & Extractive. Metall. Rev., v.25, pp.129–142.
Panda, R., Kumari, A., Jha, M. Hait, J. Kumar, V., Kumar, J., Lee J. (2014) Leaching of rare earth metals (REMs) from Korean monazite concentrate. Jour. Indian Eng. Chem., v.20, pp.2035–2042.
Rahmati, A., Ghaemi, A., Samadfam, M. (2012) Kinetic and thermodynamic studies of uranium(VI) adsorption using Amberlite IRA-910 resin. Ann. Nucl. Energy v.39, pp.42–48.
Yemel’yanov, V.S. and Yevstyukhin, A.I. (1969) The Metallurgy of Nuclear Fuel Properties and Principles of the Technology of Uranium, Thorium and Plutonium. Pergamon Press Ltd. Published by Elsevier Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borai, E.H., Hamed, M.M. & Shahr El-Din, A.M. A new method for processing of low-grade monazite concentrates. J Geol Soc India 89, 600–604 (2017). https://doi.org/10.1007/s12594-017-0649-0
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12594-017-0649-0