Abstract
During cancer chemotherapy, drugs such as 5-HT3 receptor antagonists have typically been used to control vomiting and anorexia. We examined the effects of oxytocin (OXT), which has been linked to appetite, on cisplatin-induced anorexia in rats. Fos-like immunoreactivity (Fos-LI) expressed in the supraoptic nucleus (SON), the paraventricular nucleus (PVN), the area postrema and the nucleus of the solitary tract (NTS) after intraperitoneal (ip) administration of cisplatin. We also examined the fluorescence intensity of OXT-mRFP1 after ip administration of cisplatin in OXT-mRFP1 transgenic rats. The mRFP1 fluorescence intensity was significantly increased in the SON, the PVN, and the NTS after administration of cisplatin. The cisplatin-induced anorexia was abolished by OXT receptor antagonist (OXTR-A) pretreatment. In the OXT-LI cells, cisplatin-induced Fos expression in the SON and the PVN was also suppressed by OXTR-A pretreatment. These results suggested that central OXT may be involved in cisplatin-induced anorexia in rats.
Similar content being viewed by others
Introduction
During chemotherapy for cancer, it is important to control anorexia, nausea, and vomiting. Typically, drugs such as 5-HT3 receptor antagonists [1], steroids [2], and neurokinin 1 receptor antagonists [3] are used. However, these drugs cannot entirely control anorexia and nausea during chemotherapy.
Cisplatin is widely used in chemotherapy for cancer. It exerts anti-cancer effects by inhibiting the replication of DNA [4]. Cisplatin also has various side effects, such as anorexia, nausea, and vomiting. It has been suggested that serotonin receptors are involved in the occurrence of nausea and vomiting from the use of cisplatin [1]. However, cisplatin-induced anorexia and nausea cannot be completely managed using 5-HT3 receptor antagonists [1]. Some previous studies showed that peripheral administration of cisplatin activated the neurons of various areas in the brain [5,6,7,8]. Cisplatin may also act through another pathway related to the mechanisms of anorexia and nausea.
The neurohypophysial hormone oxytocin (OXT) is mainly synthesized in the supraoptic nucleus (SON) and the paraventricular nucleus (PVN) of the hypothalamus. OXT is involved in reproduction, antinociception, anxiety, feeding, social recognition, and stress responses [9,10,11,12,13,14,15,16,17,18]. OXT has an anorexic effect that is thought to play a role in signaling satiety. Intracerebroventricular (icv) administration of OXT inhibited feeding in hungry rats, while this inhibition of feeding was prevented by co-administration of an oxytocin receptor antagonist [19, 20]. Although the site of the anorectic action of OXT is not completely understood, OXT neurons in the PVN project to the medullary dorsal nucleus of the vagus nerve, and microinjection of OXT into the dorsal nucleus of the vagus nerve was found to inhibit gastric motility, suggesting that OXT neurons of the PVN projecting to the medulla oblongata may act to inhibit feeding [21].
We have succeeded in generating transgenic rats bearing an OXT-monomeric red fluorescent protein 1 (mRFP1) fusion gene to readily visualize OXT and quantitatively evaluate OXT expression in the hypothalamus [22, 23]. Moreover, we can also use OXT-mRFP1 transgenic rats to examine OXTergic pathways from the hypothalamus to the brainstem by confirming the presence of mRFP1-positive granules in the axon terminals of OXT-expressing neurons, because OXT neurons project from the dorsal division of the parvocellular PVN (dpPVN) to the nucleus of the solitary tract (NTS) [24, 25].
In the present study, we examined the relationship between cisplatin-induced anorexia and oxytocin. First, we used Fos immunohistochemistry to examine induction of Fos-like immunoreactivity (LI) in the SON, the PVN, the area postrema (AP), and the NTS after peripheral administration of cisplatin. We also examined induction of Fos-LI in OXT neurons in the SON and the PVN by double immunostaining for Fos and OXT. The mRFP1 fluorescence intensity, which is a quantitative indicator of OXT, was also measured in the SON and the PVN in OXT-mRFP1 transgenic rats after administration of cisplatin. Moreover, mRFP1-positive granules in the NTS, which were present in the axon terminals from OXT neurons in the dpPVN, were counted manually to demonstrate the up-regulation of an OXTergic pathway from the hypothalamus to the brainstem. We also examined the effects of pretreatment with an OXT receptor antagonist (OXTR-A) on food and water intake in rats with cisplatin-induced anorexia. Finally, we used immunohistochemistry for Fos to examine the effects of pretreatment with OXTR-A on Fos-LI expression in the SON, the PVN, the AP, and the NTS after administration of cisplatin. We also examined the effects of pretreatment with OXTR-A on Fos-LI expression in the OXT neurons in the SON and the PVN using double immunostaining for Fos and OXT.
Materials and methods
Animals
We used adult male Wistar rats (weighing 200–325 g) in the experiment to measure food and water intake and to express Fos-LI. We also used adult male OXT-mRFP1 Wistar transgenic rats (weighing 220–480 g) in the fluorescence immunohistochemistry and mRFP1 fluorescence experiments. The rats were housed in plastic cages under standard conditions in an animal room at 23–25 °C with a 12:12-h light:dark cycle (lights on at 7:00 a.m.). The animals were fed a standard rat diet and tap water ad libitum. All procedures performed in this study involving animals were in accordance with the ethical standards of our institution or practice at which the studies were conducted under the control of the Ethics Committee of Animal Care and Experimentation, University of Occupational and Environmental Health, Japan. OXT-mRFP1 transgenic rats were screened by polymerase chain reactions using genomic DNA extracted from ear biopsies, as described previously [22, 23].
Reagents
Cisplatin (cis-diammineplatinum(II) dichloride) (P-4394, Sigma-Aldrich Japan Co. LLC., Tokyo, Japan) was dissolved in 0.9% sterile physiological saline (Otsuka Pharmaceutical Co. LTD., Tokyo, Japan) (0.6 mg/ml). An OXT receptor antagonist (OXTR-A) (L-368899, Tocris Bioscience, Bristol, UK) was dissolved in 0.9% sterile physiological saline (150 ng/μl).
Surgical procedures
For icv administration of solutions, animals were implanted with stainless-steel cannulae in the lateral ventricle. The animals were anesthetized (sodium pentobarbital, 50 mg/kg body weight, ip injection) and then placed in a stereotaxic frame. A stainless-steel guide cannula (550 µm outer diameter, 10 mm length) was implanted stereotaxically at the following coordinates: 0.8 mm posterior to the bregma, 1.4 mm lateral to the midline, and 2.0 mm below the surface of the left cortex, such that the tip of the cannula was 1.0 mm above the left cerebral ventricle [26]. Two stainless-steel anchoring screws were fixed to the skull, and the cannula was secured in place by acrylic dental cement. The animals were then returned to their cages and allowed to recover for at least 5 days. They were maintained in the same cages and handled daily until the start of the experiments. As they did not indicate painful behaviors any more during handling, we did not provide post-surgical analgesic treatment for them as well as previous study [27].
Central administration of OXT receptor antagonist (OXTR-A)
For icv administration of OXTR-A (150 ng/μl) or vehicle (sterile 0.9% saline), a stainless-steel injector (300 µm, outer diameter) was introduced through the cannulae at a depth of 1.0 mm beyond the end of the guide. The total volume of OXTR-A and saline solution injected into the lateral ventricle was 5 µl.
Experimental procedures
Fos immunohistochemistry and double immunohistochemistry for Fos and oxytocin
Wistar rats were intraperitoneally (ip) administered with cisplatin (6 mg/kg) or vehicle (n = 6 in each group). All rats were allowed free access to tap water and dry food. Ninety minutes after ip administration of the solution, the animals were anesthetized deeply (pentobarbital sodium, 50 mg/kg body weight ip). They were then perfused transcardially with 100 ml of 0.1 M phosphate buffer (PB; pH 7.4) containing heparin (1000 U/l) and 150 ml of a fixative containing 4% paraformaldehyde (PFA). The brains and pituitaries were carefully removed and the brains were divided into three blocks that included the hypothalamus. The brains were postfixed with 4% PFA for 48 h at 4 °C. The tissues were then cryoprotected in 20% sucrose in 0.1 M PB for 24 h at 4 °C. For immunostaining, serial sections (30 μm thick) were cut using a microtome (REM-700; Yamato Kohki Industrial Co., Ltd., Saitama, Japan). The sections were rinsed twice with 0.1 M phosphate buffered saline (PBS), after which they were washed in 0.1 M Tris buffer (pH 7.6) containing 0.3% Triton X-100. Floating sections were incubated with 1% hydrogen peroxide for 60 min, followed by a rabbit polyclonal anti-Fos protein antiserum (#sc-52, Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted at 1:500 in 0.1 M PBS containing 0.3% Triton X-100 at 4 °C for 4 days. After being washed in 0.3% Triton X-100/PBS for 20 min, the floating sections were further incubated for 120 min with a biotinylated secondary antibody solution (1:250) and finally with an avidin–biotin peroxidase complex (Vector Laboratories Inc., Burlingame, CA, USA) for 120 min. The peroxidase reaction was visualized by incubating the sections in Tris buffer containing 0.02% diaminobenzidine (DAB) and 0.05% hydrogen peroxidase for 3 min. For OXT immunostaining, the floating sections were sequentially incubated with rabbit anti-OXT antibody diluted at 1:5000 in 0.1 M PBS containing 0.3% Triton X-100 at 4 °C for 5 days. The avidin–biotin peroxidase complex was visualized with nickel sulfate-enhanced DAB. Fos-LI cells were identified by their dark brown nuclei, whereas OXT-LI cells were identified by their violet cytoplasmic and axonal precipitate. The sections were mounted on gelatin-coated glass slides and then air-dried, dehydrated in 100% ethanol, cleared with xylene, and covered with a coverslip. The number of Fos-LI and OXT-LI cells of the bilateral hypothalamic area (SON and PVN) and the medulla (AP and NTS) was counted in six sections selected according to coordinates given in a rat brain atlas [26], and the counts were averaged within each rat.
Observation of OXT-mRFP1 fluorescence in transgenic rats
OXT-mRFP1 transgenic rats were divided into six groups: control, 0, 12, 24, 48 h, and 1 week after administration of cisplatin (n = 3–6 in each group). All rats were allowed free access to tap water and dry food. The rats were deeply anesthetized with ip administration of sodium pentobarbital (50 mg/kg). They were perfused transcardially with 0.1 M phosphate buffer (PB, pH 7.4) containing heparin (1000 U/l), which was followed by 4% paraformaldehyde (PFA) in 0.1 M PB. The brains and pituitaries were carefully removed, and the brains were divided into three blocks including the hypothalamus.
The blocks were post-fixed with 4% PFA in 0.1 M PB for 48 h at 4 °C as described previously [22,23,24]. The tissues were then cryoprotected in 20% sucrose in 0.1 M PB for 48 h at 4 °C. The fixed tissues were cut at a thickness of 30 µm with a microtome (REM-700; Yamato Kohki Industrial Co., Ltd., Saitama, Japan) into sections containing the SON and PVN. The locations of the regions were determined according to coordinates given in the rat brain atlas [26]. The sections were rinsed twice with 0.1 M PB and placed on glass slides.
The sections containing the SON and PVN were examined using fluorescence microscopy (ECLIPSE Ti-E (FL/DIC); Nikon Corp., Tokyo) with an mRFP1 filter (Nikon Corp.) to examine OXT-mRFP1 expression. The images were captured with a digital camera (DS-Qi1Mc; Nikon Corp.). We selected three sections of the SON and PVN per rat. The bilateral SON and PVN were observed in the selected sections by fluorescence microscopy.
Measurement of mRFP1 fluorescence intensity in the SON, the PVN, and the NTS
The averages of mRFP1 fluorescence intensity values per unit area in the SON and the PVN were quantified with an imaging analysis system. Sections containing the SON, the PVN, and the NTS were examined using a fluorescence microscope (ECLIPSE E600; Nikon Corp., Tokyo, Japan) with an RFP filter (Nikon Corp.) in order to examine OXT-mRFP1 expression. The images were captured with a digital camera (DS-L2, DS-Fi1; Nikon Corp.). We used an imaging analysis system (NIS-Elements; Nikon Corporation, Tokyo, Japan) to average the mRFP1 fluorescence intensity per unit area in the SON and the PVN for each rat. The number of mRFP1-positive granules was counted manually in the NTS.
Measurement of water and food intake
Wistar rats were divided into four groups (n = 5–6 in each group). Five days after the implantation of stainless-steel cannulae, all rats were allowed free access to tap water and dry food. All rats had been deprived of food and free access to tap water for 24 h before experiment. Thirty minutes after icv administration of OXTR-A or saline (vehicle), we administered cisplatin or saline (vehicle). The four groups were vehicle/vehicle, vehicle/cisplatin, OXTR-A/vehicle, and OXTR-A/cisplatin. Each rat was housed in a plastic cage to measure its food and water intake 2 and 24 h after ip administration of cisplatin or vehicle.
Pretreatment with OXTR-A in Fos immunohistochemistry
Wistar rats were divided into four groups (n = 6–7 in each group). Seven days after the implantation of stainless-steel cannulae, all rats were allowed free access to tap water and dry food. Thirty minutes after icv administration of OXTR-A or saline (vehicle), we administered cisplatin or saline (vehicle). The four groups were vehicle/vehicle, vehicle/cisplatin, OXTR-A/vehicle, and OXTR-A/cisplatin. Ninety minutes after ip administration of cisplatin or vehicle, the animals were anesthetized deeply (pentobarbital sodium, 50 mg/kg body weight ip).
They were then perfused transcardially with 100 ml of 0.1 M phosphate buffer (PB; pH 7.4) containing heparin (1000 U/l) and 150 ml of a fixative containing 4% paraformaldehyde (PFA). As described in the above section, after being post-fixed, tissues were cut to a thickness of 30 µm with a microtome (REM-700; Yamato Kohki Industrial Co., Ltd., Saitama, Japan) into sections containing the SON, the PVN, and the NTS. We detected and counted Fos-LI and OXT-LI cells using immunohistochemistry for Fos and OXT, as described above.
Statistical analysis
All of the data points are presented as the mean ± SEM. Comparisons with the saline or control groups (mean ± SEM) were performed from the results of Fos expression and mRFP1 fluorescence intensity. Each group was compared with the corresponding saline or control group. The data were analyzed by one-way ANOVA with the Tukey–Kramer corrections for multiple comparisons. p < 0.05 was considered statistically significant.
Results
Fos expression in the SON, PVN, AP, and NTS
Fos-LI cells were observed in the SON, the PVN, the AP, and the NTS after ip administration of cisplatin (Fig. 1A). Fos-LI cells were scarce in all areas after ip administration of vehicle (Fig. 1A-a–e). After ip administration of cisplatin, the number of Fos-LI cells significantly increased in the SON (Fig. 1A-a), the PVN (Fig. 1A-b), the AP (Fig. 1A-c), the medial NTS (Fig. 1A-d) and the caudal NTS (Fig. 1A-e) in comparison with vehicle group.
The number of Fos-like immunoreactivity (LI) cells in the supraoptic nucleus (SON) (A-a), the paraventricular nucleus (PVN) (A-b), the area postrema (AP) (A-c), the medial nucleus of the solitary tract (mNTS) (A-d) and the caudal NTS (cNTS) (A-e) after intraperitoneal (ip) administration of cisplatin (A) in rats. Colocalization of Fos-LI (brown in round structures) and OXT-LI (violet in spindle-shaped structures) in the SON (B-a–d) and the PVN (C-a–d). B-b, d, C-b, d enlargements from the boxed areas in B-a, c and C-a, c. Black arrowheads indicate colocalization of nuclear Fos-LI and OXT-LI. White arrowheads indicate OXT-LI without Fos-LI. The asterisks indicate Fos-LI without OXT-LI. The number of Fos-expressing in OXT-LI cells in the SON (B-e) and the PVN (C-e). Values represent the mean ± SEM (each group, n = 6). *p < 0.05 compared with the vehicle group. OC optic chiasma; 3V third ventricle. Scale bars 100 μm
Fos-LI cells were also observed in OXT-LI cells in the SON (Fig. 1B-c, d) and the PVN (Fig. 1C-c, d). Few Fos-LI cells were observed in OXT-LI cells in the SON (Fig. 1B-a, b) and the PVN (Fig. 1C-a, b) after ip administration of vehicle. In OXT-LI cells, the number of Fos-LI cells significantly increased in the SON (Fig. 1B-e) and the PVN (Fig. 1C-e) in comparison with the vehicle group after ip administration of cisplatin.
mRFP1 fluorescence intensity in the SON, the PVN and the NTS of the OXT-mRFP1 transgenic rats
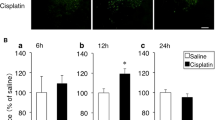
Twelve hours after ip administration of cisplatin, the average OXT-mRFP1 fluorescence intensity was significantly increased in the SON (Fig. 2A-a, B-b) and the PVN (Fig. 2A-b, B-f) compared with the control (c), 0H (0 h) and 1 week (1w) groups.
Oxytocin (OXT)-monomeric red fluorescent protein 1 (mRFP1) in the supraoptic nucleus (SON) (A-a, B-a–d), the paraventricular nucleus (PVN) (A-b, B-e–h) and the medial nucleus of the solitary tract (mNTS) (A-c, B-i–l) 0, 12, 24, and 48 h and 1 week after administration of cisplatin. The average mRFP1 fluorescence intensities are shown for the SON (A-a) and the PVN (A-b). The average number of mRFP1-positive granules in the mNTS (A-c). Values represent the mean ± SEM (each group, n = 3–6). *p < 0.05 and **p < 0.01 compared with the control group (c). # p < 0.05 and ## p < 0.01 compared with 0 h after administration of cisplatin group (0 h). $ p < 0.05 compared with 1 week after administration of cisplatin group (1w). Sections were obtained from the SON (B-a–d), PVN (B-e–h), and mNTS (B-i–l) at 0, 12, and 24 h and 1 week after administration of cisplatin. The mRFP1-positive granules are apparent (C-i–l, boxed regions). OC optic chiasma; 3V third ventricle; AP area postrema; CC central canal. Scale bar 100 μm
Twenty-four hours after ip administration of cisplatin, the number of mRFP1-positive granules was significantly increased in the mNTS compared with the control (c), 0H (0 h), and 1-week (1w) groups (Fig. 2A-c, B-k).
Effects of pretreatment with OXTR-A on food and water intake
In the vehicle/cisplatin group, food intake was significantly decreased in comparison with the vehicle/vehicle and OXTR-A/cisplatin groups 2 h after administration of solutions (2H) (Fig. 3A-a). Twenty-four hours after administration of solutions (24H), food intake in the vehicle/cisplatin and OXTR-A/cisplatin groups was significantly decreased in comparison with the vehicle/vehicle and OXTR-A/vehicle groups (Fig. 3A-b).
Food intake (A) and water intake (B) 2 h (2H) (A-a, B-a) and 24 h (24H) (A-b, B-b) after administration of cisplatin in rats. Values represent the mean ± SEM (each group, n = 5–6). *p < 0.05 and **p < 0.01 compared with the vehicle/vehicle group. # p < 0.05 and ## p < 0.01 compared with the OXTR-A/vehicle group. $ p < 0.05 compared with the OXTR-A/cisplatin group
In the vehicle/cisplatin group, water intake was significantly decreased in comparison with the vehicle/vehicle group 2 h after administration of solutions (2H) (Fig. 3B-a). Twenty-four hours after administration of solutions (24H), water intake in the vehicle/cisplatin and OXTR-A/cisplatin groups was significantly decreased in comparison with the vehicle/vehicle and OXTR-A/vehicle groups (Fig. 3A-b).
Effects of pretreatment with OXTR-A in Fos expression
Many Fos-LI cells were observed in the SON (Fig. 4A-a), the PVN (Fig. 4B-a), the AP (Fig. 4C-a), and the NTS (Fig. 4C-b, c) in the vehicle/cisplatin group. Some Fos-LI cells were observed in the SON and the PVN in the OXTR-A/cisplatin group. Few Fos-LI cells were observed in the AP and the medial NTS (mNTS) in the OXTR-A/cisplatin group. Many Fos-LI cells were observed in the caudal NTS (cNTS) in the OXTR-A/cisplatin group. Fos-LI cells were scarce in all areas in the vehicle/vehicle and OXTR-A/vehicle groups. The number of Fos-LI cells was significantly increased in the vehicle/cisplatin group in comparison with the vehicle/vehicle and OXTR-A/vehicle groups in the SON (Fig. 4A-a). Fos-LI was significantly increased in the vehicle/cisplatin group in comparison with the vehicle/vehicle, OXTR-A/vehicle and OXTR-A/cisplatin groups in the PVN (Fig. 4B-a), the AP (Fig. 4C-a) and the mNTS (Fig. 4C-b). In the cNTS, Fos-LI was significantly increased in the vehicle/cisplatin and OXTR-A/cisplatin groups in comparison with the vehicle/vehicle and OXTR-A/vehicle groups (Fig. 4C-c).
The number of Fos-like immunoreactivity (LI) cells in the supraoptic (SON) nucleus (A-a), the paraventricular nucleus (PVN) (B-a), the area postrema (AP) (C-a), the medial nucleus of the solitary tract (mNTS) (C-b) and the caudal NTS (cNTS) (C-c) after administration of cisplatin with pretreatment of oxytocin receptor antagonist (OXTR-A) pretreatment in rats. The number of Fos- expressingon in OXT-LI cells in the SON (A-b) and the PVN (B-b). Values represent the mean ± SEM (each group, n = 6–7). *p < 0.05 and **p < 0.01 compared with the vehicle/vehicle group. # p < 0.05 and ## p < 0.01 compared with the OXTR-A/vehicle group. $ p < 0.05 and $$ p < 0.01 compared with the OXTR-A/cisplatin group
In the OXT-LI cells, Fos-LI was significantly increased in the vehicle/cisplatin group in comparison with the vehicle/vehicle, OXTR-A/vehicle and OXTR-A/cisplatin groups in the SON (Fig. 4A-b) and the PVN (Fig. 4B-b).
Discussion
The present study demonstrated that Fos-LI cells in the SON, the PVN, and the NTS were significantly increased after cisplatin-induced anorexia. It also demonstrated that the mRFP1 intensity in the SON, the PVN, and the NTS was significantly increased after administration of cisplatin. Moreover, the cisplatin-induced anorexia was significantly abolished by pretreatment with OXTR-A 2 h after administration of cisplatin. Cisplatin-induced Fos expression in the SON, the PVN, the AP, and the mNTS was also significantly diminished by pretreatment with OXTR-A. This is the first study showing that central OXT may be involved in cisplatin-induced anorexia in rats.
Previous studies showed that peripheral administration of cisplatin induced Fos expression in the AP and the NTS in ferret [28, 29], least shrew [30], rat [6, 31,32,33,34], and Suncus murinus [5]. Horn et al. also reported the presence of Fos-LI cells in the SON and the PVN 48 h after administration of cisplatin in rats [31]. In the present study, we confirmed the presence of Fos-LI cells in the SON, the PVN, the AP, and the NTS after ip administration of cisplatin in rats. Moreover, we identified that Fos-LI colocalized with OXT in the SON and the PVN after administration of cisplatin and that cisplatin-induced Fos expression was diminished by pretreatment with OXTR-A. Cisplatin-induced anorexia was also inhibited by pretreatment with OXTR-A. As OXT is a well-known anorexic neuropeptide, central OXT may partially underlie cisplatin-induced anorexia.
In our present study, we can presume a mechanism about cisplatin-induced anorexia with an OXT pathway (Fig. 5). Peripherally administered cisplatin activates neurons at the cNTS via vagus nerve and subsequently activates noradrenaline neurons at the A1 and A2 area in the medulla. Activated noradrenaline neurons project to OXT neurons in the SON and the PVN. OXT neurons are activated by the autocrine and paracrine system [35]. OXT neurons in the dpPVN projected to the AP and the mNTS and induced vomiting and nausea. OXT-RA may block in the SON, the PVN, and the projection from the PVN to the AP and the mNTS. Horn demonstrated that cisplatin-induced Fos expression was diminished in the caudal NTS and part of the medial NTS, but not in the remaining part of the medial NTS or in the rostral NTS, after vagotomy [6]. Therefore, peripherally administered cisplatin induces Fos expression in the caudal and part of the medial NTS by vagal input, which comes to the caudal NTS. It is plausible that cisplatin may act, in part, on abdominal vagal afferent fibers and that its stimulus is transmitted to the caudal NTS, which is the primary location for the vagal afferent fiber synapses that transmit satiety information from the gastrointestinal system [36, 37]. Some previous studies have reported the existence of a neuronal projection from the NTS to the SON and the PVN through noradrenergic pathways, resulting in anorexia [38, 39]. Maejima et al. demonstrated that OXT stimulates pro-opiomelanocortin (POMC) neurons, which synthesize bioactive peptides such as α-melanocyte-stimulating hormone (MSH) in the NTS [40]. Melanocortin-4 receptors (MC4R), which are involved in feeding, expressed in the SON and the PVN [41,42,43,44,45]. In light of our results, we propose that ip-administered cisplatin may primarily affect vagal afferent fibers and activate NTS–PVN noradrenergic projections onto OXT neurons in the PVN. OXT from dpPVN may be carried to POMC neurons in the medial NTS, and α-MSH from POMC neurons may activate the neurons of the SON and the PVN, which express MC4R and relate to feeding. In the present study, pretreatment with OXTR-A significantly mitigated cisplatin-induced anorexia and Fos expression in the SON, the PVN, the AP, and the medial NTS, but not the caudal NTS. These results may support our hypothesis (Fig. 5).
A possible pathway of activated oxytocin (OXT) neurons after peripherally administered cisplatin. Peripherally administered cisplatin activates neurons at the caudal nucleus of the solitary tract (cNTS) via vagus nerve and subsequently activates noradrenaline (NA) neuron at the A1 and A2 area in the medulla. Activated → NA neurons project to OXT neurons in the supraoptic (SON) nucleus and the paraventricular nucleus (PVN). OXT neurons are activated by the autocrine and paracrine system. OXT neurons in the PVN project to the area postrema (AP) and the medial NTS (mNTS), and induced → nausea and vomiting. OXT receptor antagonist (OXTR-A) may block the autocrine and paracrine system in the SON and the PVN, and the projection from the PVN to the AP and the mNTS
In the present study, 12 h after administration of cisplatin, the average OXT-mRFP1 fluorescence intensity was significantly increased in the SON and the PVN compared with the control. Meanwhile, the number of mRFP1-positive granules did not change in the mNTS 12 h after administration of cisplatin, and it was significantly increased in the mNTS 24 h after administration of cisplatin. OXT neurons in the dpPVN project to the AP and the medial NTS. Therefore, peripheral administered cisplatin may stimulate neurons in the SON and the PVN; subsequently OXT neurons were activated by autocrine and paracrine, and this stimulus is transmitted from the dpPVN to the medial NTS (Fig. 5). There may be a time lag in the visualization of mRFP1; when we investigate the mRFP1 intensity at a finer temporal resolution, we may be able to observe the change fully. This point should be examined in further research.
We demonstrated that OXT may be involved with cisplatin-induced anorexia and Fos expression in the SON, the PVN, the AP, and the medial NTS in the present study. Delta-9-tetrahydrocannabinol, a specific cannabinoid-1 receptor antagonist, inhibited cisplatin-induced anorexia and emesis and diminished cisplatin-induced Fos expression in the AP and NTS in ferrets [29] and in the least shrew [30]. Palonosetron, a 5-HT3 receptor antagonist, diminished cisplatin-induced Fos expression in the NTS in the Suncus murinus [5]. Therefore, peripherally administered cisplatin induces anorexia and activates neurons through mechanisms related to several neuropeptides, including OXT.
OXT is released from the posterior pituitary. Although we did not measure plasma OXT levels after ip administration of cisplatin, in a preliminary study, the mRFP1 intensity was decreased in the posterior pituitary in OXT-mRFP1 transgenic rats 24 h after ip administration of cisplatin (data not shown). Although we did not measure plasma OXT levels, we can presume that peripherally administered cisplatin may activate OXT neurons in the SON and the PVN and release OXT from the posterior pituitary into circulation. However, changes in mRFP1 intensity may not always parallel changes in plasma oxytocin levels. Thus, we need to measure plasma OXT levels after peripheral administration of cisplatin in a future study.
Although cisplatin-induced anorexia was significantly attenuated by pretreatment with OXTR-A at 2 h after administration of cisplatin, these effects of OXTR-A were abolished at 24 h after administration of cisplatin. As the half-valued period of L-368899 was about 2 h [46], we presumed that the effects of L-368899 would keep only for several hours. Therefore, cisplatin may have been induced anorexia after the ineffective with L-368899.
Our used OXTR-A, L-368899, is non-specific to the OXT receptor. Our used OXTR-A may block AVP V1a and V2 receptors. Although we did not investigate the effects of arginine vasopressin (AVP) after peripherally administered cisplatin, cisplatin may be involved in the AVP system in the hypothalamus. These points should be examined in further research.
In conclusion, we demonstrated that the numbers of Fos-LI cells in the SON, PVN, and NTS were significantly increased after peripherally administered cisplatin. We also demonstrated that the mRFP1 intensity in the SON, the PVN, and the NTS was significantly increased after peripherally administered cisplatin. Moreover, cisplatin-induced anorexia was abolished by pretreatment with OXTR-A. Cisplatin-induced Fos expressions in the SON, the PVN, and the NTS were also significantly diminished by pretreatment with OXTR-A. These results suggest that central OXT may be involved in cisplatin-induced anorexia in rats.
References
Percie du Sert N, Rudd JA, Apfel CC, Andrews PL (2011) Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT (3) receptor antagonists. Cancer Chemother Pharmacol 67:667–686
Shinkai T, Saijo N, Eguchi K, Sasaki Y, Tamura T, Fujiwara Y, Mae M, Fukuda M, Ohe Y, Sasaki S et al (1989) Control of cisplatin-induced delayed emesis with metoclopramide and dexamethasone: a randomized controlled trial. Jpn J Clin Oncol 19:40–44
Ruhlmann C, Herrstedt J (2009) Casopitant: a novel NK(1)-receptor antagonist in the prevention of chemotherapy-induced nausea and vomiting. Ther Clin Risk Manag 5:375–384
Kocsis F, Klein W, Altmann H (1973) A screening system to determine inhibition of specific enzymes of the semiconservative DNA-synthesis and DNA-repair replication (author’s transl). Zeitschrift fur Naturforschung Teil C Biochemie Biophysik Biologie 28:131–135
De Jonghe BC, Horn CC (2009) Chemotherapy agent cisplatin induces 48-h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol 296:902–911
Horn CC (2009) Brain Fos expression induced by the chemotherapy agent cisplatin in the rat is partially dependent on an intact abdominal vagus. Auton Neurosci 148:76–82
Horn CC, De Jonghe BC, Matyas K, Norgren R (2009) Chemotherapy-induced kaolin intake is increased by lesion of the lateral parabrachial nucleus of the rat. Am J Physiol Regul Integr Comp Physiol 297:1375–1382
Holland RA, Leonard JJ, Kensey NA, Hannikainen PA, De Jonghe BC (2014) Cisplatin induces neuronal activation and increases central AMPA and NMDA receptor subunit gene expression in mice. Physiol Behav 136:79–85
Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM (1987) Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab 64:27–31
Carmichael MS, Warburton VL, Dixen J, Davidson JM (1994) Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav 23:59–79
Russell J, Leng G (1998) Sex, parturition and motherhood without oxytocin? J Endocrinol 157:342–359
Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, Anderson GM, Riddle MA, McDougle CJ, Barr LC, Donald J, Cohen DJ (1994) The role of central oxytocin in obsessive compulsive disorder and related normal behavior. Psychoneuroendocrinology 19:723–749
Stock S, Uvnas-Moberg K (1988) Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiol Scand 132:29–34
Uvnäs-Moberg K, Bruzelius G, Alster P, Lundeberg T (1993) The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand 149:199–204
Higashida H, Lopatina O, Yoshihara T, Pichugina YA, Soumarokov AA, Munesue T, Minabe Y, Kikuchi M, Ono Y, Korshunova N, Salmina AB (2010) Oxytocin signal and social behaviour: comparison among adult and infant oxytocin, oxytocin receptor and CD38 gene knockout mice. J Neuroendocrinol 22:373–379
Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR (2008) Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol 22:1723–1734
Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y (2008) Oxytocin and appetite. Prog Brain Res 170:137–151
Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA (2006) Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav 5:274–281
Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG (1991) Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12:113–118
Verbalis JG, Blackburn RE, Olson BR, Stricker EM (1993) Central oxytocin inhibition of food and salt ingestion: a mechanism for intake regulation of solute homeostasis. Regul Pept 45:149–154
Verbalis JG, Blackburn RE, Hoffman GE, Stricker EM (1995) Establishing behavioral and physiological functions of central oxytocin: insights from studies of oxytocin and ingestive behaviors. Adv Exp Med Biol 395:209–225
Katoh A, Fujihara H, Ohbuchi T, Onaka T, Hashimoto T, Kawata M, Suzuki H, Ueta Y (2011) Highly visible expression of an oxytocin-monomeric red fluorescent protein 1 fusion gene in the hypothalamus and posterior pituitary of transgenic rats. Endocrinology 52:2768–2774
Katoh A, Shoguchi K, Matsuoka H, Yoshimura M, Ohkubo JI, Matsuura T, Maruyama T, Ishikura T, Aritomi T, Fujihara H, Hashimoto H, Suzuki H, Murphy D, Ueta Y (2014) Fluorescent visualisation of the hypothalamic oxytocin neurones activated by cholecystokinin-8 in rats expressing c-fos-enhanced green fluorescent protein and oxytocin-monomeric red fluorescent protein 1 fusion transgenes. J Neuroendocrinol 26:341–347
Matsuura T, Kawasaki M, Hashimoto H, Ishikura T, Yoshimura M, Ohkubo JI, Maruyama T, Motojima Y, Sabanai K, Mori T, Ohnishi H, Sakai A, Ueta Y (2015) Fluorescent visualisation of oxytocin in the hypothalamo-neurohypophysial/-spinal pathways after chronic inflammation in oxytocin-monomeric red fluorescent protein 1 transgenic rats. J Neuroendocrinol 27:636–646
Motojima Y, Kawasaki M, Matsuura T, Saito R, Yoshimura M, Hashimoto H, Ueno H, Maruyama T, Suzuki H, Ohnishi H, Sakai A, Ueta Y (2016) Effects of peripherally administered cholecystokinin-8 and secretin on feeding/drinking and oxytocin-mRFP1 fluorescence in transgenic rats. Neurosci Res 109:63–69
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier Academic Press, Amsterdam
Hashimoto H, Hyodo S, Kawasaki M, Mera T, Chen L, Soya A, Saito T, Fujihara H, Higuchi T, Takei Y, Ueta Y (2005) Centrally administered adrenomedullin 2 activates hypothalamic oxytocin-secreting neurons, causing elevated plasma oxytocin level in rats. Am J Physiol Endocrinol Metab 289(5):E753–E761
Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H (2000) The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett 286:123–126
Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA (2003) Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol 285:566–576
Ray AP, Griggs L, Darmani NA (2009) Delta 9-tetrahydrocannabinol suppresses vomiting behavior and Fos expression in both acute and delayed phases of cisplatin-induced emesis in the least shrew. Behav Brain Res 196:30–36
Horn CC, Ciucci M, Chaudhury A (2007) Brain Fos expression during 48 hours after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci 132:44–51
Batra VR, Schrott LM (2011) Acute oxycodone induces the pro-emetic pica response in rats. J Pharmacol Exp Ther 339:738–745
Cui Y, Wang L, Shi G, Liu L, Pei P, Guo J (2016) Electroacupuncture alleviates cisplatin-induced nausea in rats. Acupunct Med 34:120–126
De Jonghe BC, Holland RA, Olivos DR, Rupprecht LE, Kanoski SE, Hayes MR (2016) Hindbrain GLP-1 receptor mediation of cisplatin-induced anorexia and nausea. Physiol Behav 153:109–114
Ludwig M, Stern J (2015) Multiple signalling modalities mediated by dendritic exocytosis of oxytocin and vasopressin. Philos Trans R Soc Lond B Biol Sci 370. doi:10.1098/rstb.2014.0182
De Jonghe BC, Horn CC (2008) Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol 294:756–765
Berthoud HR, Neuhuber WL (2000) Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 85:1–17
Cunningham ET Jr, Sawchenko PE (1988) Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 274:60–76
Ueta Y, Kannan H, Higuchi T, Negoro H, Yamaguchi K, Yamashita H (2000) Activation of gastric afferents increases noradrenaline release in the paraventricular nucleus and plasma oxytocin level. J Auton Nerv 78:69–76
Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh-I S, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T (2009) Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10:355–365
Sabatier M, Pont F, Arnaud MJ, Turnlund JR (2003) A compartmental model of magnesium metabolism in healthy men based on two stable isotope tracers. Am J Physiol Regul Integr Comp Physiol 285:656–663
Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW (1997) Melanocortin receptors in leptin effects. Nature 390:349
Shah BP, Vong L, Olson DP, Koda S, Krashes MJ, Ye C, Yang Z, Fuller PM, Elmquist JK, Lowell BB (2014) MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc Natl Acad Sci USA 111:13193–13198
Siljee JE, Unmehopa UA, Kalsbeek A, Swaab DF, Fliers E, Alkemade A (2013) Melanocortin 4 receptor distribution in the human hypothalamus. Eur J Endocrinol 168:361–369
Singru PS, Wittmann G, Farkas E, Zséli G, Fekete C, Lechan RM (2012) Refeeding-activated glutamatergic neurons in the hypothalamic paraventricular nucleus (PVN) mediate effects of melanocortin signaling in the nucleus tractus solitarius (NTS). Endocrinology 153:3804–3814
Thompson KL, Vincent SH, Miller RR, Colletti AE, Alvaro RF, Wallace MA, Feeney WP, Chiu SH (1997) Pharmacokinetics and disposition of the oxytocin receptor antagonist L-368,899 in rats and dogs. Drug Metab Dispos 25(10):1113–1118
Acknowledgements
We would like to thank Ms. Kanako Shoguchi and Ms. Yuki Nonaka (University of Occupational and Environmental Health, Kitakyushu, Japan) for their technical assistance. This study was supported by Grant-in-Aid for Scientific Research (C) number 16K08537 from the Japan Society for the Promotion of Science (JSPS), the Japan Agency for Medical Research and Development (AMED), and a research grant from Mitsubishi Tanabe Pharma Corporation.
Author information
Authors and Affiliations
Contributions
KA: study design/experimental studies/figure preparation/manuscript preparation/statistical analysis; HH: study design/experimental studies/figure preparation/manuscript preparation/statistical analysis/manuscript review; SS: experimental studies/manuscript editing; HU: experimental studies/manuscript editing; RS: experimental studies/manuscript editing; YM: experimental studies/manuscript editing; MY: manuscript editing/experimental studies/manuscript review; TM: manuscript editing; KH: study design/guarantor of integrity of the study/manuscript editing; YU: study design/guarantor of integrity of the study; YU: study design/guarantor of integrity of the study/manuscript review/final approval.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a conflict of interest to disclose.
Funding
This study was funded by Grant-in-Aid for Scientific Research (C) number 16K08537 from the Japan Society for the Promotion of Science (JSPS), the Japan Agency for Medical Research and Development (AMED), a and research grant from Mitsubishi Tanabe Pharma Corporation.
Ethical approval
All procedures performed in this study involving animals were in accordance with the ethical standards of our institution or practice at which the studies were conducted under the control of the Ethics Committee of Animal Care and Experimentation, University of Occupational and Environmental Health, Japan.
About this article
Cite this article
Arase, K., Hashimoto, H., Sonoda, S. et al. Possible involvement of central oxytocin in cisplatin-induced anorexia in rats. J Physiol Sci 68, 471–482 (2018). https://doi.org/10.1007/s12576-017-0550-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-017-0550-z