Abstract
Background
Although several echo-Doppler methods were proposed to optimize atrioventricular (AV) delay in patients with sequential ventricular pacing, “echo-guided” AV optimization has not been widely adopted clinically. A combination of trasmitral flow (TMF) and pulmonary venous flow (PVF) measurements may be beneficial to further optimize AV delay to achieve better cardiac function. The aim of this study was to assess the feasibility and usefulness of AV delay optimization by combined use of TMF and PVF.
Methods
A total of 32 patients after sequential ventricular pacemaker implantation were enrolled and studied. The optimal AV delay was defined as the timing to minimize the duration between PVF reversal (a) wave and the duration of the “A” wave of TMF. Stroke volume was measured at the “optimized” AV delay (AVDOPT) and was compared with that obtained at shorter (AVDOPT − 50 ms) and longer (AVDOPT + 50 ms) AV delays.
Results
AV optimization was feasible in 27 of 32 patients (87 %). Stroke volume at AVDOPT was significantly higher than that at shorter or longer AV delay (63 ± 18 ml vs. 57 ± 15 ml vs. 56 ± 16 ml, P = 0.001).
Conclusions
AV delay optimization using TMF and PV flow was feasible. Usefulness of this method requires further investigation with a larger study population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Left ventricular (LV) dysfunction may develop as a result of LV dyssynchrony and/or inappropriate atrioventricular (AV) delay in some patients after single chamber, ventricular pacing. Even after dual-chamber sequential pacing, maintenance of AV synchrony is necessary to preserve cardiac function and to achieve a better prognosis [1, 2]. AV delay optimization is, therefore, important to maintain better cardiac function and a favorable long-term outcome after sequential pacing [3, 4] or cardiac resynchronization therapy [5, 6]. Although several echo-Doppler- [7–13] as well as electrocardiogram- [14–17] based methods to optimize AV interval have been proposed, routine or systematic use of AV optimization remains controversial [5, 6, 18–20]. Transmitral flow (TMF) by transthoracic Doppler echocardiography is commonly used to optimize AV delay. However, the advantage of echo-Doppler-based AV optimization over fixed AV delay or a commercially available AV optimization algorithm based on electrocardiogram has not been proven yet.
Theoretically (based on the Frank–Starling law), AV delay should be optimized to achieve maximal LV filling without deterioration of LV function [2]. Because TMF alone does not reflect both systolic function and LV filling pressure, TMF-based AV optimization may not provide enough advantage over the other methods. A previous echo-Doppler study demonstrated that the difference between the duration of pulmonary venous flow reversal (PVa) and mitral forward flow during atrial systole (A) reliably estimates LV filling pressure [21]. We hypothesized that a combination of TMF and PV flow measurements may be beneficial to further optimize AV delay to achieve better cardiac function with adequate LV filling pressure. Therefore, the aim of this study was to assess feasibility of the AV delay optimization by combined use of TMF and PV flow.
Materials and methods
Study population
This study included 32 patients after dual-chamber pacing for complete AV block (n = 26, mean age = 79 ± 8 years; 12 males) or cardiac resynchronization therapy (n = 6, mean age = 65 ± 16 years; 4 males). The exclusion criteria were current atrial arrhythmia and frequent premature ventricular beats. Informed consent was provided by each participant before enrollment in this study.
Study protocol
Echocardiography was performed with a Sonos 5500 and S3 transducer (Philips Medical Systems, Andover, MA, USA). TMF was obtained from apical 3-chamber or 4-chamber views with the sample volume positioned at the tip of the mitral leaflets. TMF consists of 2 distinct flow signals, early or E wave and late or A wave during atrial contraction. PV flow was obtained from an apical 4-chamber view with the sample volume placed in the left superior pulmonary vein. An effort was made to maintain the same position of the pulsed Doppler sample throughout the echo-Doppler examination.
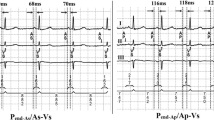
AV delay optimization was performed using TMF and PV flow at rest. Optimal AV delay (AVDOPT) was defined as the AV delay where the duration of PVa minus A was the minimum (=0). Because the onset of the A wave cannot be always detected, the difference between the duration of PVa and A was alternatively measured as (time interval between the onset of the Q wave and the end of the A wave) − (time interval between the onset of the Q wave and the end of the PVa wave). To simplify this method, TMF and PV flow were recorded at the pre-set AV delay. Then, AVDOPT was determined as (pre-set AV delay) + (duration of PVa − duration of A) (Fig. 1). Stroke volume (SV) was measured by a pulsed Doppler method obtained at the LV outflow tract and was used as an index to assess cardiac function during AV optimization. SV obtained at the AVDOPT was compared with SV obtained at shorter (AVDOPT − 50 ms) or longer (AVDOPT + 50 ms) AV delays.
AV delay optimization using TMF and PV flow. a (Step 1) At a pre-set AV delay (=180 ms in this case), both TMF and PV flow signal were recorded. (Step 2) The difference in duration between PVa and A wave was measured (=−30 ms). b (Step 3) Optimal AV delay was calculated as (pre-set AV delay) + (duration of PVa − duration of A). In this case, the optimal AV delay was calculated as 180 ms + (−30 ms) = 150 ms
Statistical analysis
The measurements are expressed as mean ± standard deviation. Statistical analyses were performed with one-way analysis of variance (ANOVA) using the Bonferroni post hoc test. Values of P < 0.05 were considered statistically significant.
Results
Clinical characteristics of the 32 patients are shown in Table 1. Dual chamber (DDD) pacing was used in 12 patients and ventricular (VDD) pacing in 20 patients. All patients after dual-chamber sequential pacing were in New York Heart Association (NYHA) class I or П. On the other hand, all patients after cardiac resynchronization therapy (CRT) were in NYHA class III. Left ventricular ejection fraction (LVEF) was 55 ± 15 %.
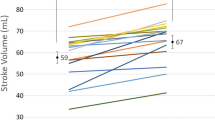
AV optimization using our current method could be performed in 27 of 32 patients (84 %). In the remaining 5 patients, adequate PV flow signal could not be recorded. The measurements made in all patients are summarized in Tables 2 and 3. Mean AVDOPT was 143 ± 35 ms. As expected, the mean AVDOPT was significantly lower in the VDD than in the DDD mode (133 ± 32 ms vs. 170 ± 37 ms, P = 0.014). SV at AVDOPT was significantly higher than shorter or longer AV delay (63 ± 18 ml vs. 57 ± 15 ml vs. 56 ± 16 ml, P = 0.001) (Fig. 2).
In a subset of patients after sequential dual-chamber pacing for complete AV block, AV optimization could be performed in 22 of 26 patients (85 %). The AVDOPT in VDD mode was 128 ± 38 ms and the AVDOPT in the DDD pacing mode was 177 ± 39 ms. SV with AVDOPT was significantly higher than shorter or longer AV delay (64 ± 19 ml vs. 57 ± 16 ml vs. 56 ± 17 ml, P = 0.0001) (Fig. 3).
Similarly, in a subset of patients after CRT, AV optimization could be performed in 5 of 6 patients (83 %). The AVDOPT in VDD mode was 128 ± 38 ms and the AVDOPT in the DDD pacing mode was 177 ± 39 ms. SV with AVDOPT was significantly higher than shorter or longer AV delay (61 ± 13 ml vs. 53 ± 11 ml vs. 57 ± 10 ml, P = 0.026) (Fig. 4).
Reproducibility of PV flow measurements was analyzed. Correlation coefficients were high for repeated measurements by the same observer (r = 0.98 for duration of PVa minus A) and measurements by 2 different observers (r = 0.88 for duration of PVa minus A).
Discussion
This study shows that AV delay optimization based on a new echo-Doppler method using TMF and PV flow is feasible. In addition, increased SV during AV delay optimized by this method may suggest a potential favorable impact on cardiac function and possibly prognosis.
A previous randomized, prospective study comparing echo-guided AV delay optimization and an empiric, fixed AV delay of 120 ms demonstrated improved clinical outcome at 3 months in patients with echo-guided AV optimization [19]. In their study, optimal AV delay was defined as the largest aortic velocity–time integral at one of eight tested AV intervals (between 60 and 200 ms). On the other hand, a more recent large-scale randomized prospective multicenter trial (SMART-AV trial) to compare between a fixed empirical AV delay (120 ms), echocardiographically optimized AV delay, and AV delay optimized with SmartDelay electrocardiogram-based algorithm did not show superiority of echocardiography or SmartDelay over a fixed AV delay of 120 ms [18]. In their study, Ritter’s method [10, 22] and/or an iterative method [23] using TMF were used to optimize AV delay as endorsed by the American Society of Echocardiography [23, 24]. Based on their negative results, the authors stated that routine echocardiographic AV optimization using the American Society of Echocardiography recommended method for patients with CRT should be abandoned [18]. However, it is not certain whether all echo-Doppler methods should be abolished.
Ritter et al. [22] first reported an echo-Doppler method to optimize AV delay in patients with complete AV block and a normal LV systolic function. Ritter et al. defined the AV delay with the echo method that provided the longest diastolic filling time without interruption of the A wave. Ritter’s formula, which can be regarded as the current “gold standard” in AV delay optimization [24] requires 2 measurements: (1) QA short = the time interval between the onset of the Q wave and the end of the truncated “A” wave of the TMF at a short (30–60 ms) AV delay; and (2) QA long = the time interval between the onset of the Q wave and the end of the “A” wave of the TMF at a long (200 ms) AV interval. According to the formula, optimal AV delay was calculated as AV long − (QA short − QA long). This method has been used in several clinical trials because it is a simple, non-invasive and reproducible method [20]. On the other hand, Ishikawa et al. used diastolic mitral regurgitation to optimize AV delay. As compared with Ritter’s method in which AV delay was optimized to achieve the highest cardiac output, Ishikawa’s method is to achieve the lowest possible left atrial or LV filling pressure [9, 25]. In our present study, we used both TMF and PV flow to achieve the lowest LV filling pressure and the highest SV.
The concept of Doppler assessment of LV filling pressure using both TMF and PV flow was first reported in 1993 by Rossvoll and Hatle [21]. The difference in duration between PVa of the PV flow and antegrade A wave by the TMF was positively and strongly correlated with LV end-diastolic pressure (r = 0.68, P < 0.001). A longer duration of PVa versus A wave predicted increased (>15 mmHg) LV end-diastolic pressure [21]. The mechanisms for a longer duration of PVa than the A wave was explained by the increased LV end-diastolic pressure as a result of reduced LV compliance. Therefore, an AV delay that does not prolong PVa more than the A wave could be considered as a hemodynamically optimal AV delay. Although our preliminary data suggest that AV optimization based on the TMF and PV flow is feasible, it was not possible for AV optimization to be performed in some patients in whom PV flow could not be detected. This is a possible limitation of this study. Detection of the PV flow signal using the transthoracic approach depends upon the image quality of the echo-Doppler machine. Although the sensitivity of the Doppler measurements for some specific conditions was not sufficient when using old echo-Doppler machines and therefore required contrast enhancement [26, 27], modern echo-Doppler machines have sufficiently sensitive Doppler equipment [28, 29]. Another apparent limitation is that 2 different Doppler measurements are required for our method which appears to be time consuming. However, as compared with Ritter’s method, which requires 2 TMF recordings at 2 different AV delay settings, our method is less time consuming.
Because this is a small pilot study, further investigations will be necessary. First, the hemodynamically favorable acute results should be confirmed by invasive hemodynamic monitoring. Second, the long-term clinical impact of the acute results should be investigated by a serial observation of the study population. Finally, the advantages of the current method should be investigated by comparing it with other echo-Doppler methods or empirical fixed AV delay prospectively.
Conclusions
A novel AV delay optimization method using TMF and PV flow has been shown to be feasible. The usefulness of this method requires further investigation with a larger study population.
References
Frielingsdorf J, Gerber AE, Hess OM. Importance of maintained atrio-ventricular synchrony in patients with pacemakers. Eur Heart J. 1994;15:1431–40.
Guardigli G, Ansani L, Percoco GF, et al. AV delay optimization and management of DDD paced patients with dilated cardiomyopathy. Pacing Clin Electrophysiol. 1994;17:1984–8.
Pearson AC, Janosik DL, Redd RR, et al. Doppler echocardiographic assessment of the effect of varying atrioventricular delay and pacemaker mode on left ventricular filling. Am Heart J. 1988;115:611–21.
Toda N, Ishikawa T, Nozawa N, Kobayashi I, et al. Doppler index and plasma level of atrial natriuretic hormone are improved by optimizing atrioventricular delay in atrioventricular block patients with implanted DDD pacemakers. Pacing Clin Electrophysiol. 2001;24:1660–3.
Daubert JC, Saxon L, Adamson PB, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–86.
Whinnett ZI, Davies JE, Willson K, et al. Haemodynamic effects of changes in atrioventricular and interventricular delay in cardiac resynchronisation therapy show a consistent pattern: analysis of shape, magnitude and relative importance of atrioventricular and interventricular delay. Heart. 2006;92:1628–34.
Tei C, Nishimura RA, Seward JB, et al. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78.
Gola A, Pozzoli M, Capomolla S, et al. Comparison of Doppler echocardiography with thermodilution for assessing cardiac output in advanced congestive heart failure. Am J Cardiol. 1996;78:708–12.
Ishikawa T, Sumita S, Kimura K, et al. Prediction of optimal atrioventricular delay in patients with implanted DDD pacemakers. Pacing Clin Electrophysiol. 1999;22:1365–71.
Ritter P, Padeletti L, Gillio-Meina L, et al. Determination of the optimal atrioventricular delay in DDD pacing. Comparison between echo and peak endocardial acceleration measurements. Europace. 1999;1:126–30.
Meluzin J, Novak M, Mullerova J, et al. A fast and simple echocardiographic method of determination of the optimal atrioventricular delay in patients after biventricular stimulation. Pacing Clin Electrophysiol. 2004;27:58–64.
Jansen AH, Bracke FA, van Dantzig JM, et al. Correlation of echo-Doppler optimization of atrioventricular delay in cardiac resynchronization therapy with invasive hemodynamics in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97:552–7.
Morales MA, Startari U, Panchetti L, et al. Atrioventricular delay optimization by doppler-derived left ventricular dP/dt improves 6-month outcome of resynchronized patients. Pacing Clin Electrophysiol. 2006;29:564–8.
Gervais R, Leclercq C, Shankar A, et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11:699–705.
Khaykin Y, Exner D, Birnie D, et al. Adjusting the timing of left-ventricular pacing using electrocardiogram and device electrograms. Europace. 2011;13:1464–70.
Kamdar R, Frain E, Warburton F, et al. A prospective comparison of echocardiography and device algorithms for atrioventricular and interventricular interval optimization in cardiac resynchronization therapy. Europace. 2010;12:84–91.
Ishikawa T, Sugano T, Sumita S, et al. Optimal atrioventricular delay setting determined by evoked QT interval in patients with implanted stimulus-T-driven DDDR pacemakers. Europace. 2001;3:46–51.
Ellenbogen KA, Gold MR, Meyer TE, et al. Primary results from the smart delay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122:2660–8.
Sawhney NS, Waggoner AD, Garhwal S, et al. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004;1:562–7.
Antonini L, Auriti A, Pasceri V, et al. Optimization of the atrioventricular delay in sequential and biventricular pacing: physiological bases, critical review, and new purposes. Europace. 2012;14:929–38.
Rossvoll O, Hatle LK. Pulmonary venous flow velocities recorded by transthoracic Doppler ultrasound: relation to left ventricular diastolic pressures. J Am Coll Cardiol. 1993;21:1687–96.
Ritter P, Dib JC, Lelievre T, et al. Quick determination of the optimal AV delay at rest in patients paced in DDD mode for complete AV block. Eur JCPE. 1994;4:163.
Kedia N, Ng K, Apperson-Hansen C, et al. Usefulness of atrioventricular delay optimization using Doppler assessment of mitral inflow in patients undergoing cardiac resynchronization therapy. Am J Cardiol. 2006;98:780–5.
Gorcsan J 3rd, Abraham T, Agler DA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213.
Ishikawa T, Sumita S, Kimura K, et al. Critical PQ interval for the appearance of diastolic mitral regurgitation and optimal PQ interval in patients implanted with DDD pacemakers. Pacing Clin Electrophysiol. 1994;17:1989–94.
Izumi C, Yoshida K, Akasaka T, et al. Improved visualization of pulmonary venous flow Doppler signal by intravenous injection of sonicated albumin. Am J Cardiol. 1996;78:598–600.
Okura H, Yoshida K, Akasaka T, et al. Improved transvalvular continuous-wave Doppler signal intensity after intravenous albunex injection in patients with prosthetic aortic valves. J Am Soc Echocardiogr. 1997;10:608–12.
Okura H, Fuyuki H, Kubo T, et al. Noninvasive diagnosis of ischemic and nonischemic cardiomyopathy using coronary flow velocity measurements of the left anterior descending coronary artery by transthoracic Doppler echocardiography. J Am Soc Echocardiogr. 2006;19:552–8.
Okura H, Takada Y, Yamabe A, et al. Prevalence and correlates of physiological valvular regurgitation in healthy subjects. Circ J. 2011;75:2699–704.
Conflict of interest
Kenzo Fukuhara, Hiroyuki Okura, Terumasa Koyama, Teruyoshi Kume, Yoji Neishi, Akihiro Hayashida, Kiyoshi Yoshida declare that they have no conflict of interest.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Fukuhara, K., Okura, H., Koyama, T. et al. Feasibility of a novel atrioventricular delay optimization method using transmitral and pulmonary venous flow in patients with sequential ventricular pacing or cardiac resynchronization therapy. J Echocardiogr 13, 52–58 (2015). https://doi.org/10.1007/s12574-014-0237-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-014-0237-x