Abstract
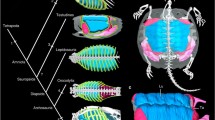
The evolution of the turtle shell has long been one of the central debates in comparative anatomy. The turtle shell consists of dorsal and ventral parts: the carapace and plastron, respectively. The basic structure of the carapace comprises vertebrae and ribs. The pectoral girdle of turtles sits inside the carapace or the rib cage, in striking contrast to the body plan of other tetrapods. Due to this topological change in the arrangement of skeletal elements, the carapace has been regarded as an example of evolutionary novelty that violates the ancestral body plan of tetrapods. Comparing the spatial relationships of anatomical structures in the embryos of turtles and other amniotes, we have shown that the topology of the musculoskeletal system is largely conserved even in turtles. The positional changes seen in the ribs and pectoral girdle can be ascribed to turtle-specific folding of the lateral body wall in the late developmental stages. Whereas the ribs of other amniotes grow from the axial domain to the lateral body wall, turtle ribs remain arrested axially. Marginal growth of the axial domain in turtle embryos brings the morphologically short ribs in to cover the scapula dorsocaudally. This concentric growth appears to be induced by the margin of the carapace, which involves an ancestral gene expression cascade in a new location. These comparative developmental data allow us to hypothesize the gradual evolution of turtles, which is consistent with the recent finding of a transitional fossil animal, Odontochelys, which did not have the carapace but already possessed the plastron.





Similar content being viewed by others
References
Aoyama H, Asamoto K (2000) The developmental fate of the rostral/caudal half of a somite for vertebra and rib formation: experimental confirmation of the resegmentation theory using chick-quail chimeras. Mech Dev 99:71–82
Aoyama H, Mizutani-koseki S, Koseki H (2005) Three developmental compartments involved in rib formation. Int J Dev Biol 49:325–333
Birchmeier C, Brohmann H (2000) Genes that control the development of migrating muscle precursor cells. Curr Opin Cell Biol 12:725–730
Braun T, Arnold HH (1995) Inactivation of Myf-6 and Myf-5 genes in mice leads to alterations in skeletal muscle development. EMBO J 14:1176–1186
Braun T, Bober E, Rudnicki MA, Jaenisch R, Arnold HH (1994) MyoD expression marks the onset of skeletal myogenesis in Myf-5 mutant mice. Development 120:3083–3092
Braun T, Rudnicki MA, Arnold HH, Jaenisch R (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71:369–382
Brent AE, Braun T, Tabin CJ (2005) Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 132:515–528
Brent AE, Schweitzer R, Tabin CJ (2003) A somitic compartment of tendon progenitors. Cell 18:235–248
Burke AC, Nowicki JL (2003) A new view of patterning domains in the vertebrate mesoderm. Dev Cell 4:159–165
Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121:333–346
Burke AC (1989) Development of the turtle carapace: implications for the evolution of a novel bauplan. J Morphol 199:363–378
Burke AC (1991) The development and evolution of the turtle body plan. Inferring intrinsic aspects of the evolutionary process from experimental embryology. Am Zool 31:616–627
Burke AC (2009) Turtles…again. Evol Dev 11:622–624
Carroll RL (1988) Vertebrate paleontology and evolution. Freeman, New York
Carus KG (1834) Lehrbuch der Vergleichenden Zootomie, vol 1, 2nd edn. Fleischer, Leipzig
Cebra-Thomas JA, Betters E, Yin M, Plafkin C, McDow K, Gilbert SF (2007) Evidence that a late-emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta. Evol Dev 9:267–277
Cebra-Thomas J, Tan F, Sistla S et al (2005) How the turtle forms its shell: a paracrine hypothesis of carapace formation. J Exp Zool 304B:558–569
Christ B, Jacob HJ, Jacob M (1974) Origin of wing musculature. Experimental studies on quail and chick embryos. Experientia 30:1446–1449
Christ B, Jacob M, Jacob HJ (1983) On the origin and development of the ventrolateral abdominal muscles in the avian embryo: an experimental and ultrastructural study. Anat Embryol 166:87–101
Christ B, Wilting J (1992) From somites to vertebral column. Ann Anat 174:23–32
Claessens LPAM (2004) Dinosaur gastralia; origin, morphology, and function. J Vertebr Paleontol 24:89–106
Clark K, Bender G, Murray BP et al (2001) Evidence for the neural crest origin of turtle plastron bones. Genesis 31:111–117
Collins CA, Watt FM (2008) Dynamic regulation of retinoic acid-binding proteins in developing, adult and neoplastic skin reveals roles for β-catenin and notch signalling. Dev Biol 324:55–67
Cuvier G (1800) Leçons d’Anatomie Comparée, vol 1. Boudouin, Paris
Danilkovitch-Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ (2001) Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the β-catenin pathway. Mol Cell Biol 21:5857–5868
Evans DJR (2003) Contribution of somitic cells to the avian ribs. Dev Biol 256:114–126
Evans DJ, Valasek P, Schmidt C, Patel K (2006) Skeletal muscle translocation in vertebrates. Anat Embryol 211:43–50
Fraidenraich D, Iwahori A, Rudnicki M, Basilico C (2000) Activation of fgf4 gene expression in the myotomes is regulated by myogenic bHLH factors and by sonic hedgehog. Dev Biol 225:392–406
Fraidenraich D, Lang R, Basilico C (1998) Distinct regulatory elements govern Fgf4 gene expression in the mouse blastocyst, myotomes, and developing limb. Dev Biol 204:197–209
Gaffney ES (1990) The comparative osteology of the Triassic turtle Proganochelys. Bull Am Mus Nat Hist 194:1–263
Gegenbaur C (1898) Vergleichende Anatomie der Wirbelthiere. Engelmann, Leipzig
Geoffroy Saint-Hilaire E (1818) Philosophie Anatomique, vol 1. Baillière, Paris
Gilbert SC (2010) Developmental biology, 9th edn. Sinauer, Sunderland
Gilbert SF, Loredo GA, Brukman A, Burke AC (2001) Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol Dev 3:47–58
Gilbert SF, Bender G, Betters E, Yin M, Cebra-Thomas JA (2007) The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell. Integr Comp Biol 47:401–408
Gilbert SF, Cebra-Thomas JA, Burke AC (2008) How the turtle gets its shell. In: Wyneken J, Godfrey MH, Bels V (eds) Biology of turtles. CRC, Boca Raton, pp 1–16
Goette A (1899) Über die Entwicklung des knöchernen Ruckenschildes (Carapax) der Schildkröten. Z Wiss Zool 66:407–434
Goodrich ES (1930) Studies on the structure and development of vertebrates. McMillan, London
Grass S, Arnold HH, Braun T (1996) Alterations in somite patterning of Myf-5-deficient mice: a possible role for FGF-4 and FGF-6. Development 122:141–150
Haeckel E (1895) Systematische Phylogenie. Entwurf eines Natürlichen Systems der Organismen auf Grund ihrer Stammesgeschichte. Reimer, Berlin
Hall BK (1998) Evolutionary developmental biology, 2nd edn. Chapman & Hall, London
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–91
Huang R, Zhi Q, Neubüser A et al (1996) Function of somite and somitocoele cells in the formation of the vertebral motion segment in avian embryos. Acta Anat 155:231–241
Huang R, Zhi Q, Schmidt C, Wilting J, Brand-Saberi B, Christ B (2000a) Sclerotomal origin of the ribs. Development 127:527–532
Huang R, Zhi Q, Scmhidt C, Brand-Saberi B, Christ B (2000b) New experimental evidence for somite resegmentation. Anat Embryol 202:195–200
Huang R, Zhi Q, Wilting J, Christ B (1994) The fate of the somitocoele cells in avian embryos. Anat Embryol 190:243–250
Joyce WG, Lucas SG, Scheyer TM, Heckert AB, Hunt AP (2009) A thin-shelled reptile from the Late Triassic of North America and the origin of the turtle shell. Proc R Soc Lond B276:507–513
Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA (1997) MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development 124:4729–4738
Kato N, Aoyama H (1998) Dermomyotomal origin of the ribs as revealed by extirpation and transplantation experiments in chick and quail embryos. Development 125:3437–3443
Kawashima-Ohya Y, Narita Y, Nagashima H, Usuda U, Kuratani S (2011) Hepatocyte growth factor is crucial for development of the carapace in turtles. Evol Dev 13:260–268
Kuraku S, Usuda R, Kuratani S (2005) Comprehensive survey of carapacial ridge-specific genes in turtle implies co-option of some regulatory genes in carapace evolution. Evol Dev 7:3–17
Kuratani S, Kuraku S, Nagashima H (2011) Evolutionary developmental perspective for the origin of the turtles: the folding theory for the shell based on the developmental nature of the carapacial ridge. Evol Dev 13:1–14
Kusakabe R, Kuratani S (2005) Evolution and developmental patterning of the vertebrate skeletal muscles: perspectives from the lamprey. Dev Dyn 234:824–834
Kusakabe R, Kuratani S (2007) Evolutionary perspectives from development of mesodermal components in the lamprey. Dev Dyn 236:410–420
Kälin J (1945) Zur Morphogenese des Panzers bei den Schildkröten. Acta Anat 1:144–176
Lee MSY (1993) The origin of the turtle body plan: bridging a famous morphological gap. Science 261:1716–1720
Lee MSY (1996) Correlated progression and the origin of turtles. Nature 379:812–815
Li C, Wu X, Rieppel O, Wang L, Zhao L (2008) An ancestral turtle from the Late Triassic of southwestern China. Nature 45:497–501
Loredo GA, Brukman A, Harris MP et al (2001) Development of an evolutionarily novel structure: fibroblast growth factor expression in the carapacial ridge of turtle embryos. J Exp Zool 291B:274–281
Monga SP, Mars WM, Pediaditakis P et al (2002) Hepatocyte growth factor induces Wnt-independent nuclear translocation of β-catenin after Met-β-catenin dissociation in hepatocytes. Cancer Res 62:2064–2071
Moustakas JE (2008) Development of the carapacial ridge: implications for the evolution of genetic networks in turtle shell development. Evol Dev 10:29–36
Nagashima H, Kuraku S, Uchida K, Ohya YK, Narita Y, Kuratani S (2007) On the carapacial ridge in turtle embryos: its developmental origin, function, and the chelonian body plan. Development 134:2219–2226
Nagashima H, Sugahara F, Takechi M et al (2009) Evolution of the turtle body plan by the folding and creation of new muscle connections. Science 325:193–196
Nagashima H, Uchida K, Yamamoto K, Kuraku S, Usuda R, Kuratani S (2005) Turtle-chicken chimera: an experimental approach to understanding evolutionary innovation in the turtle. Dev Dyn 232:149–161
Nagashima H, Kuraku S, Uchida K, Kawashima-Ohya Y, Narita Y, Kuratani S (in press) Origin of the turtle body plan—the folding theory to illustrate turtle-specific developmental repatterning. In: Brinkman DB, Holroyd PA, Gardner JD (eds) Morphology and evolution of turtles: origin and early diversification. Springer, Dordrecht
Nelson WJ, Nusse R (2004) Convergence of Wnt, β-catenin, and cadherin pathways. Science 303:1483–1487
Nowicki JL, Burke AC (2000) Hox genes and morphological identity: axial versus lateral patterning in the vertebrate mesoderm. Development 127:4265–4275
Nowicki JL, Takimoto R, Burke AC (2003) The lateral somitic frontier: dorso-ventral aspects of anterio-posterior regionalization in avian embryos. Mech Dev 120:227–240
Ogushi K (1911) Anatomische Studien an der japanischen dreikralligen Lippenschildkröte (Trionyx japonicus). Morphol Jahrb 43:1–106
Ohya YK, Kuraku S, Kuratani S (2005) Hox code in embryos of Chinese soft-shelled turtle Pelodiscus sinensis correlates with the evolutionary innovation in the turtle. J Exp Zool 304B:107–118
Ohya YK, Usuda R, Kuraku S, Nagashima H, Kuratani S (2006) Unique features of Myf-5 in turtles: nucleotide deletion, alternative splicing and unusual expression pattern. Evol Dev 8:415–423
Olivera-Martinez I, Coltey M, Dhouailly D, Pourqui O (2000) Mediolateral somitic origin of ribs and dermis determined by quail-chick chimeras. Development 127:4611–4617
Olson EN, Arnold HH, Rigby PWJ, Wold BJ (1996) Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell 85:1–4
Owen FRS (1849) On the development and homologies of the carapace and plastron of the chelonian reptiles. Philos Trans R Soc Lond 139:151–171
Parker WK (1868) A monograph on the structure and development of the shoulder-girdle and sternum in the Vertebrata. Hardwicke, London
Patapoutian A, Yoon JK, Miner JH, Wang S, Stark K, Wold B (1995) Disruption of the mouse MRF4 gene identifies multiple waves of myogenesis in the myotome. Development 121:3347–3358
Pinot M (1969) Etude expérimentale de la morphogenése de la cage thoracique chez l’embryon de poulet: mécanismes et origine du matériel. J Embryol Exp Morph 21:149–164
Rasola A, Fassetta M, De Bacco F et al (2007) A positive feedback loop between hepatocyte growth factor receptor and β-catenin sustains colorectal cancer cell invasive growth. Oncogene 26:1078–1087
Rathke H (1848) Ueber die Entwickelung der Schildkröten. Vieweg, Braunschweig
Rieppel O (2001) Turtles as hopeful monsters. BioEssays 23:987–991
Rieppel O (2009) How did the turtle get its shell? Science 325:154–155
Rieppel O (in press) The evolution of the turtle shell. In: Brinkman DB, Holroyd PA, Gardner JD (eds) Morphology and evolution of turtles: origin and early diversification. Springer, Dordrecht
Romer AS (1956) Osteology of the reptiles. University of Chicago Press, Chicago
Romer AS, Parsons TS (1977) The vertebrate body. Saunders, Philadelphia
Ruckes H (1929) Studies in chelonian osteology part II, the morphological relationships between the girdles, ribs and carapace. Ann NY Acad Sci 31:81–120
Saunders JW Jr (1948) The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool 108:363–403
Saunders JW Jr, Reuss C (1974) Inductive and axial properties of prospective wing-bud mesoderm in the chick embryo. Dev Biol 38:41–50
Scheyer TM, Brüllmann B, Sánchez-Villagra MR (2008) The ontogeny of the shell in side-necked turtles, with emphasis on the homologies of costal and neural bones. J Morphol 269:1008–1021
Seno T (1961) An experimental study on the formation of the body wall in the chick. Acta Anat 45:60–82
Sensenig EC (1949) The early development of the human vertebral column. Contrib Embryol 33:23–40
Shearman RM, Burke AC (2009) The lateral somitic frontier in ontogeny and phylogeny. J Exp Zool 312B:603–612
Shimomura Y, Agalliu D, Vonica A et al (2010) APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature 464:1043–1047
Sudo H, Takahashi Y, Tonegawa A et al (2001) Inductive signals from the somatopleure mediated by bone morphogenetic proteins are essential for the formation of the sternal component of avian ribs. Dev Biol 232:284–300
Summerbell D, Lewis JH, Wolpert L (1973) Positional information in chick limb morphogenesis. Nature 224:492–496
Sweeney RM, Watterson RL (1969) Rib development in chick embryos analyzed by means of tantalum foil blocks. Am J Anat 126:127–150
Tabin C, Wolpert L (2007) Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Gen Dev 21:1433–1442
Takahashi M, Fujita M, Furukawa Y et al (2002) Isolation of a novel human gene, APCDD1, as a direct target of the β-catenin/T-cell factor 4 complex with probable involvement in colorectal carcinogenesis. Cancer Res 62:5651–5656
Takahashi M, Nakamura Y, Obama K, Furukawa Y (2005) Identification of SP5 as a downstream gene of the β-catenin/Tcf pathway and its enhanced expression in human colon cancer. Int J Oncol 27:1483–1487
Theißen G (2006) The proper place of hopeful monsters in evolutionary biology. Theory Biosci 124:349–369
Theißen G (2009) Saltational evolution: hopeful monsters are here to stay. Theory Biosci 128:43–51
Valasek P, Theis S, Krejci E, Grim M, Maina F, Shwartz Y et al (2010) Somitic origin of the medial border of the mammalian scapula and its homology to the avian scapula blade. J Anat 216:482–488
Valasek P, Theis S, DeLaurier A, Hinits Y, Luke GN, Otto AM et al (2011) Cellular and molecular investigations into the development of the pectoral girdle. Dev Biol 357:108–116
Vallén E (1942) Beiträge zur Kenntnis der Ontogenie und der vergleichenden Anatomie des Schildkrötenpanzers. Acta Zool 23:1–127
Vasyutina E, Birchmeier C (2006) The development of migrating muscle precursor cells. Anat Embryol 211:S37–S41
Vinagre T, Moncaut N, Carapuço M, Nóvoa A, Bom J, Mallo M (2010) Evidence for a myotomal Hox/Myf cascade governing nonautonomous control of rib specification within global vertebral domains. Dev Cell 18:655–661
Vincent C, Bontoux M, Le Douarin NM, Pieau C, Monsoro-Burq AH (2003) Msx genes are expressed in the carapacial ridge of turtle shell: a study of the European pond turtle, Emys orbicularis. Dev Genes Evol 213:464–469
Walker WF Jr (1947) The development of the shoulder region of the turtle, Chrysemys picta marginata, with special reference to the primary musculature. J Morphol 80:195–249
Wang B, He L, Ehehalt F et al (2005) The formation of the avian scapula blade takes place in the hypaxial domain of the somites and requires somatopleure-derived BMP signals. Dev Biol 287:11–18
Watson DSM (1914) Eunotosaurus africanus Seeley and the ancestors of the Chelonia. Proc Zool Soc Lond 11:1011–1020
Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT (2005) The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/β-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol 15:489–500
Winter B, Braun T, Arnold HH (1992) Co-operativity of functional domains in the muscle-specific transcription factor Myf-5. EMBO J 11:1843–1855
Yntema CL (1970) Extirpation experiments on the embryonic rudiments of the carapace of Chelydra serpentina. J Morphol 132:235–244
Yoon JK, Olson EN, Arnold HH, Wold BJ (1997) Different MRF4 knockout alleles differentially disrupt Myf-5 expression: cis-regulatory interactions at the MRF4/Myf-5 locus. Dev Biol 188:349–362
Zangerl R (1939) The homology of the shell elements in turtles. J Morphol 65:383–409
Zangerl R (1969) The turtle shell. In: Gans C, Bellairs Ad’A, Parsons TS (eds) The biology of the reptilia, vol 1. Academic Press, New York, pp 311–319
Zhang W, Behringer RR, Olson EN (1995) Inactivation of the myogenic bHLH gene MRF4 results in up-regulation of myogenin and rib anomalies. Genes Dev 9:1388–1399
Acknowledgment
We thank Dr. O. Rieppel for allowing us to cite his unpublished paper.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagashima, H., Kuraku, S., Uchida, K. et al. Body plan of turtles: an anatomical, developmental and evolutionary perspective. Anat Sci Int 87, 1–13 (2012). https://doi.org/10.1007/s12565-011-0121-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-011-0121-y