Abstract
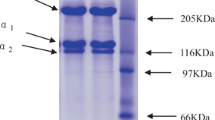
This study investigates an efficient method to extract collagen from the skins of sea bass. The ultrasonic treatment (20 kHz with amplitude of 20–80 %) was applied to fish skin for 24 h after the addition of 0.01, 0.05, and 0.1 M acetic acid (1:200 sample/acetic acid, w/v). As a result, the rate (Ki) of the collagen yield increased depending on the amount of acid added, the duration of ultrasonic treatment and the amplitude of the ultrasonic waves. The subunit compositions of the extracted components were analyzed by SDS-PAGE, and the main components were determined as collagen, including the α1 (α3), α2, β, and γ chains. And in addition to collagen, some unknown components were also observed after a longer period of ultrasonic treatment. Therefore, we had to optimize the efficient extraction conditions for pure collagen while minimizing the creation of unknown components. The most effective extraction condition for collagen by ultrasonic treatment was 80 % amplitude with 0.1 M acetic acid for 3 h of treatment. It was found that the component extracted by the ultrasonic treatment was indeed collagen, since there were no changes in the main components of collagen after pepsin treatment.






Similar content being viewed by others
References
Bracho GE, Haard NF (1990) Determination of collagen crosslinks in rockfish skeletal muscle. J Food Biochem 14:435–451
Sikorski ZE, Scott DN, Buisson DH (1984) The role of collagen in the quality and processing of fish. Crit Rev Food Sci Nutr 20:301–343
Leuenberger BH (1991) Investigation of viscosity and gelatin properties of different mammalian and fish gelatins. Food Hydrocoll 41:353–361
Kimura S (1992) Wide distribution of skin Type I collagen α3-chain in bony fish. Comp Biochem Physiol B 102:255–260
Kimura S, Miyauchi Y, Uchida N (1991) Scale and bone Type I collagens of carp (Cyprinus carpio). Comp Biochem Physiol B 99:473–476
Kimura S, Ohno Y (1987) Fish Type I collagen: tissue specific existence of two molecular forms (α1)2, α2 and α1α2α3, in Alaska pollock. Comp Biochem Physiol B 88:409–413
Ciarlo AS, Paredi ME, Fraga AN (1997) Isolation of soluble collagen from hake skin (Merluccius hubbsi). J Aquac Food product Tech 6:65–77
HO H, Lin LH, Sheu MT (1997) Characterization of collagen isolation and application of collagen gel as a drug carrier. J Controlled Release 44:103–112
John FC, Paul DF, Karl HK (1993) Collagen fabrics as biomaterials. Biotech Bioeng 43:781–791
Takeshi N, Nobutaka S (2000) Isolation of collagen from fish waste material: skin, bone and fins. Food Chem 68:277–281
Kim HK, Kim YH, Kim YJ, Park HJ, Lee NH (2012) Effects of ultrasonic treatment on collagen extraction from skins of the sea bass Lateolabrax japonicas. Fish Sci 78:485–490
Kruus P, O’Neill M, Robertson D (1990) Ultrasonic initiation of polymerization. Ultrasonics 28:304–309
AOAC Association of Official Analytical Chemists (1999) In: K. Helrich (ed) Official methods analysis, 14th edn Washington, DC
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Floros JD, Liang H (1994) Acoustically assisted diffusion through membranes and biomaterials. Food Technol 48:79–84
Crum L (1995) Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason Sonochem 2:S147–S152
Ana CS, Mar V (2010) Effect of ultrasound on the technological properties and bioactivity of food: a review. Trends Food Sci Technol 21:323–331
Lawrence SB, Mitchell RZ, Edward BF, Kenneth SS (1996) Cavitation thermometry using molecular and continuum sololuminescence. J Phys Chem 100:6612–6619
Lawrence AC (1995) Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrasonic sonochem 2:147–152
Acknowledgments
This study was supported by the research fund from Korea Food Research Institute, Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.K., Kim, Y.H., Park, H.J. et al. Application of ultrasonic treatment to extraction of collagen from the skins of sea bass Lateolabrax japonicus . Fish Sci 79, 849–856 (2013). https://doi.org/10.1007/s12562-013-0648-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-013-0648-z