Abstract
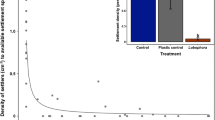
In situ larval seeding is a low-cost technique that is currently under development for the large-scale restoration of coral populations. One problem that still needs to be solved is the preparation of coral larvae for seeding, i.e., how many larvae are required to restore a certain area? In this study, we focused on the relationship between the numbers of larvae, settlers, and survivors for three months post-settlement to determine the optimal larval seeding density. A comparison of three different larval densities (low, middle, and high) indicated that the number of settlers was proportional to the larval density, suggesting that settler density is determined by the number of larvae supplied. However, the survival rate of settlers on high-density plates was much lower than the corresponding rates on low- or middle-density plates during the first month after settlement. Moreover, most of the seeded corals had not survived on the low-density plates at three months after settlement. Therefore, the middle larval density (i.e., 5000 larvae m−2) appears to be optimal for seeding on grid plates.





Similar content being viewed by others
References
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nystrom M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:9290–9933
Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortés J, Delbeek JC, DeVantier L, Edgar G, Edwards AJ, Fenner D, Guzmán HM, Hoeksema BW, Hodgson G, Johan O, Licuanan WY, Livingstone SR, Lovell ER, Moore JA, Obura DO, Ochavillo D, Polidoro BA, Precht WF, Quibilan MC, Reboton C, Richards ZT, Rogers AD, Sanciangco J, Sheppard A, Sheppard C, Smith J, Stuart S, Turak E, Veron JE, Wallace C, Weil E, Wood E (2008) One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321:560–563
Knowlton N, Jackson JBC (2006) Shifting baselines, local impacts, and global change on coral reefs. PLoS Biol 6:e54. doi:10.1371/journal.pbio.0060054
McManus JW (1997) Tropical marine fisheries and a future of coral reefs: a brief review with emphasis on Southeast Asia. Coral Reefs 16:S121–S127
Stottrup JG, Sparrevohn CR (2007) Can stock enhancement enhance stocks? J Sea Res 57:104–113
Bell JD, Leber KM, Blankenship HL, Loneragan NR, Masuda R (2008) A new era for restocking, stock enhancement and sea ranching of coastal fisheries resources. Rev Fish Sci 16:1–9
Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA (1997) Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst 27:477–500
Alexander SE, Roughgarden J (1996) Larval transport and population dynamics of intertidal barnacle: a coupled benthic/oceanic model. Ecol Monogr 66:259–275
Armsworth PR (2002) Recruitment limitation, population regulation, and larval connectivity in reef fish metapopulation. Ecology 83:1092–1104
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett JE, Tanner JE, Willis BL (1999) Patterns of recruitment and abundance of corals along the Great Barrier Reef. Nature 397:59–63
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2000) Supply-side ecology works both ways: the link between benthic adults, fecundity, and larval recruits. Ecology 81:2241–2249
Hatta M, Iwao K, Taniguchi H, Omori M (2004) Restoration technology using sexual reproduction. In: Omori M, Fujiwara S (eds) Manual for restoration and remediation of coral reefs. Nature Conservation Bureau, Ministry of the Environment, Tokyo, pp 14–28
Heyward AJ, Smith LD, Rees M, Field SN (2002) Enhancement of coral recruitment by in situ mass culture of coral larvae. Mar Ecol Prog Ser 230:113–118
Omori M (2005) Success of mass culture of Acropora corals from egg to colony in open water. Coral Reefs 24:563
Guest J, Heyward A, Omori M, Iwao K, Morse A, Boch C (2010) Rearing coral larvae for reef rehabilitation. In: Edwards AJ (ed) Reef rehabilitation manual. Coral Reef Targeted Research and Capacity Building for Management Program, St. Lucia, pp 73–92
Omori M (2011) Degradation and restoration of coral reefs: experience in Okinawa, Japan. Mar Biol Res 7:3–12
Suzuki G, Kai S, Yamashita H, Suzuki K, Iehisa Y, Hayashibara T (2011) Narrower grid structure of artificial reef enhances initial survival of in situ settled corals. Mar Pollut Bull 62:2803–2812
Ando W, Watanabe K, Tamura M, Miyake T, Kitano M, Yamamoto H (2009) Discussion about “function and structure” for the artificial base of coral distribution. Annu J Civ Eng Ocean 25:461–466 (in Japanese with English abstract)
Suzuki K, Iehisa Y, Harada T, Shibuno T, Hayashibara T, Abe O (2009) Japan Patent Kokai no. P2009-77649
Suzuki G, Arakaki S, Kai S, Hayashibara T (2012) Habitat differentiation in the early life stage of simultaneously mass-spawning corals. Coral Reefs 31:535–545
Sano M, Shimizu M, Nose Y (1987) Long-term effects of destruction of hermatypic corals by Acanthaster planci infestation on reef fish communities at Iriomote Island, Japan. Mar Ecol Prog Ser 37:191–199
Nanami A, Yamada H (2008) Foraging rates and substratum selection in foraging activity of checkered snapper Lutjanus decussatus (Lutjanidae) in an Okinawan coral reef. J Fish Biol 73:1484–1488
Hayashibara T, Iwao K, Omori M (2004) Induction and control of spawning in Okinawa staghorn corals. Coral Reefs 23:406–409
Suzuki G, Arakaki S, Hayashibara T (2011) Rapid in situ settlement following spawning by Acropora corals at Ishigaki, southern Japan. Mar Ecol Prog Ser 421:131–138
Suzuki G, Hayashibara T, Shirayama Y, Fukami H (2008) Evidence of species-specific habitat selectivity of Acropora corals based on identification of new recruits by two molecular markers. Mar Ecol Prog Ser 355:149–159
Baria MVB, Guest JR, Edwards AJ, Alino PM, Heyward AJ, Gomez ED (2010) Caging enhances post-settlement survival of juveniles of the scleractinian coral Acropora tenuis. J Exp Mar Biol Ecol 394:149–153
Christiansen NA, Ward S, Harii S, Tibbetts IR (2009) Grazing by a small fish affects the early stages of a post-settlement stony coral. Coral Reefs 28:47–51
Acknowledgments
We thank the staff of Ishigaki Tropical Station, Seikai National Fisheries Research Institute, for helping with the field research, and two anonymous reviewers for their valuable comments and suggestions. The sampling of corals was exceptionally permitted by the Okinawa Prefectural Government for research use (no. 21–40).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, G., Arakaki, S., Suzuki, K. et al. What is the optimal density of larval seeding in Acropora corals?. Fish Sci 78, 801–808 (2012). https://doi.org/10.1007/s12562-012-0504-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-012-0504-6