Abstract
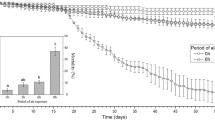
The Pacific oyster Crassostrea gigas is a sessile bivalve that inhabits the intertidal zone and therefore frequently exposed to air during the tidal cycle. It is highly adaptive to hypoxic conditions. We have studied the physiological state of oysters during long-term exposure to air. The oysters became hypoxic when exposed to air or hypoxic seawater. The 50% lethal time of oysters exposed to air at 4, 15 and 20°C was 47.8, 15.9 and 12.2 days, respectively. The hemolymph pH decreased by day 3; however, it showed a slight increase by day 5 at both 4 and 20°C. The adenylate energy charge (AEC) values decreased rapidly on the first day of air exposure in the adductor muscle, mantle, gill and body trunk, and these decreases were accompanied by decreases in ATP concentrations and increases in AMP concentrations. The AEC values in all of the tissues had fallen to below 30% by day 50 of air exposure at 4°C. These data suggest that the energy state of oysters deteriorates rapidly with air exposure. Consequently, AEC values may be useful indices of the physiological state of the oyster during long-term exposure to air.







Similar content being viewed by others
References
Widdows J, Bayne BL, Lyvingstone DR (1979) Physiological and biochemical responses of bivalve mollusks to exposure to air. Comp Biochem Physiol 62A:301–308
Zurburg W, Kluytmans JH (1980) Organ specific changes in energy metabolism due to anaerobiosis in the sea mussel Mytilus edulis (L.). Comp Biochem Physiol 67B:317–322
Zurburg W, Ebberink RHM (1981) The anaerobic energy demand of Mytilus edulis. Organ specific differences in ATP-supplying processes and metabolic routes. Mol Physiol 1:153–164
Eberlee JC, Storey JM, Storey KB (1983) Anaerobiosis, recovery from anoxia, and the role of strombine and alanopine in the oyster Crassostrea virginica. Can J Zool 61:2682–2687
Korycan SA, Storey KB (1983) Organ-specific metabolism during anoxia and recovery from anoxia in the cherrystone clam, Mercenaria mercenaria. Can J Zool 61:2674–2681
Isani G, Cattani O, Zurzolo M, Pagnucco C, Cortesi P (1995) Energy metabolism of the mussel, Mytilus galloprovincialis, during long-term anoxia. Comp Biochem Physiol 110B:103–113
Bacchiocchi S, Principato G (2000) Mitochondrial contribution to metabolic changes in the digestive gland of Mytilus galloprovincialis during anaerobiosis. J Exp Zool 286:107–113
Ballantyne JS (2004) Mitochondria: aerobic and anaerobic design—lessons from mollusks and fishes. Comp Biochem Physiol 139B:461–467
Michaelidis B, Haas D, Grieshaber MK (2005) Extracellular and intracellular acid–base status with regard to the energy metabolism in the oyster Crassostrea gigas during exposure to air. Physiol Biochem Zool 78:373–383
Moullac GL, Bacca H, Huvet A, Moal J, Pouvreau S, Wormhoudt AV (2007) Transcriptional regulation of pyruvate kinase and phosphoenolpyruvate carboxykinase in the adductor muscle of the oyster Crassostrea gigas during prolonged hypoxia. J Exp Zool 307A:371–382
Jokumsen A, Fyhn HJ (1982) The influence of aerial exposure upon respiratory and osmotic properties of haemolymph from two intertidal mussels, Mytilus edulis L and Modiolus modiolus L. J Exp Mar Biol Ecol 61:189–203
Atkinson DE (1968) The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry 7:4030–4034
Ivanovici AM (1980) Adenylate energy charge: an evaluation of applicability to assessment of pollution effects and directions for future research. Rapp P-v Réun Cons Int Explor Mer 179:23–28
Suwetja IK, Hori K, Miyazawa K, Ito K (1989) Changes in content of ATP-related compounds, homarine, and trigonelline in marine invertebrates during ice storage. Nippon Suisan Gakkaishi 55:559–566
Kawashima K, Yamanaka H (1992) Effects of storage temperatures on the post-mortem biochemical changes in scallop adductor muscle. Nippon Suisan Gakkaishi 58:2175–2180
Watanabe H, Yamanaka H, Yamakawa H (1992) Post-mortem biochemical changes in the muscle of disk abalone during storage. Nippon Suisan Gakkaishi 58:2081–2088
Yokoyama Y, Sakaguchi M, Kawai F, Kanamori M (1992) Changes in concentration of ATP-related compounds in various tissues of oyster during ice storage. Nippon Suisan Gakkaishi 58:2125–2136
Yokoyama Y, Sakaguchi M, Kawai F, Kanamori M (1994) Effects of storage temperature on postmortem changes of ATP and its related compounds and freshness indices in oyster tissues. Fish Sci 60:217–223
Yokoyama Y, Sakaguchi M, Kawai F, Kanamori M (1994) Chemical indices for assessing freshness of shellfish during storage. Fish Sci 60:329–333
Yokoyama Y, Azuma Y, Sakaguchi M, Kawai F, Kanamori M (1996) Non-destructive phosphorus-31 nuclear magnetic resonance study of post-mortem changes in oyster tissues. Fish Sci 62:416–420
Yokoyama Y, Sakaguchi M, Azuma Y, Kawai F, Kanamori M (1996) Postmortem changes of ATP and its related compounds in oyster tissues in the presence of antibiotic chloramphenicol. Fish Sci 62:312–316
Moal J, Samain JF, Le Coz JR, Daniel JY (1989) Responses and adaptations of adenylate energy charge and digestive enzyme activities to tidal emersion of Crassostrea gigas population in Marennes-Oleron Bay. Sci Mar 53:699–704
Sakaguchi M, Murata M (1989) Seasonal variations of free amino acids in oyster whole body and adductor muscle. Nippon Suisan Gakkaishi 55:2037–2041
Chiou T-K, Lai M-M, Shiau C-Y (2001) Seasonal variations of chemical constituents in the muscle and viscera of small abalone fed different diets. Fish Sci 67:146–156
Blanco SL, Suárez MP, Juan FS (2006) Seasonal changes of nucleotides in mussel (Mytilus galloprovincialis) mantle tissue. Comp Biochem Physiol 143B:384–390
Díaz-Enrich MJ, Ramos-Martínez JI, Ibarguren I (2002) Implication of guanosine 3′,5′-cyclic monophosphate, adenosine 3′,5′-cyclic monophosphate, adenosine 5′-mono-, di- and triphosphate and fructose-2, 6-bisphosphate in the regulation of the glycolytic pathway in hypoxic/anoxic mussel, Mytilus galloprovincialis. Mol Cell Biochem 240:111–118
Kawabe S, Yokoyama Y (2009) cDNA cloning and expression of grp94 in the Pacific oyster Crassostrea gigas. Comp Biochem Physiol 154B:290–297
Zurburg W, Zwaan A (1981) The role of amino acids in anaerobiosis and osmoregulation in bivalves. J Exp Zool 215:315–325
Tomanek L, Somero GN (2000) Time course and magnitude of synthesis of heat-shock proteins in congeneric marine snails (genus Tegula) from different tidal heights. Physiol Biochem Zool 73:249–256
Tomanek L, Somero GN (2002) Interspecific- and acclimation-induced variation in levels of heat-shock proteins 70 (hsp70) and 90 (hsp90) and heat-shock transcription factor-1 (HSF1) in congeneric marine snails (genus Tegula): implications for regulation of hsp gene expression. J Exp Biol 205:677–685
Snyder MJ, Girvetz E, Mulder EP (2001) Induction of marine mollusk stress proteins by chemical or physical stress. Arch Environ Contam Toxicol 41:22–29
Piano A, Asirelli C, Caselli F, Fabbri E (2002) Hsp70 expression in thermally stressed Ostrea edulis, a commercially important oyster in Europe. Cell Stress Chaperones 7:250–257
Boutet I, Tanguy A, Rousseau S, Auffret M, Moraga D (2003) Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassosrea gigas. Cell Stress Chaperones 8:76–85
Boutet I, Tanguy A, Moraga D (2004) Response of the Pacific oyster Crassostrea gigas to hydrocarbon contamination under experimental conditions. Gene 329:147–157
Hosoi M, Kubota S, Toyohara M, Toyohara H, Hayashi I (2003) Effect of salinity change on free amino acid content in Pacific oyster. Fish Sci 69:395–400
Farcy E, Serpentini A, Fiévet B, Lebel JM (2007) Identification of cDNAs encoding HSP70 and HSP90 in the abalone Haliotis tuberculata: transcriptional induction in response to thermal stress in hemocyte primary culture. Comp Biochem Physiol 146B:540–550
David E, Tanguy A, Pichavant K, Moraga D (2005) Response of the Pacific oyster Crassostrea gigas to hypoxia exposure under experimental conditions. FEBS J 272:5635–5652
Murata M, Sakaguchi M (1986) Changes in contents of free amino acids, trimethylamine, and nonprotein nitrogen of oyster during ice storage. Bull Jpn Soc Sci Fish 52:1975–1980
Ivanovici AM (1980) The adenylate energy charge in the estuarine mollusk, Pyrazus ebeninus. Laboratory studies of responses to salinity and temperature. Comp Biochem Physiol 66A:43–55
Shofer SL, Tjeerdema RS (1998) Effects of hypoxia and toxicant exposure on adenylate energy charge and cytosolic ADP concentrations in abalone. Comp Biochem Physiol 119C:51–57
Hatanaka M (1940) Chemical composition of the oyster, Ostrea gigas Thumberg. Nippon Suisan Gakkaishi 9:21–26
Kawabe S, Yokoyama Y (2010) Molecular cloning of calnexin and calreticulin in the Pacific oyster Crassostrea gigas and its expression in response to air exposure. Mar Genomics 3:19–27
Groenendyk J, Michalak M (2005) Endoplasmic reticulum quality control and apoptosis. Acta Biochim Pol 52:381–395
Acknowledgments
This work was partially supported by a grant from Fukui Prefectural University for Special Research Projects and also by a Sasakawa Scientific Research Grant from The Japan Science Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawabe, S., Takada, M., Shibuya, R. et al. Biochemical changes in oyster tissues and hemolymph during long-term air exposure. Fish Sci 76, 841–855 (2010). https://doi.org/10.1007/s12562-010-0263-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-010-0263-1