Abstract
The transmission pathway of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 also called COVID-19 disease) in indoor environments are the main area of contention between health systems and scientists. In this context, little has been investigated about the collection of airborne viral shedding. Here, we collected air samples from 24 locations inside the sole COVID-19 patient care center in Zabol, Iran, for screening SARS-CoV-2 RNA from March to May 2021. Locations included the ICU, COVID-19 wards (CWs) rooms, corridors, nearby nurses’ stations, and toilets. We identified the SARS-CoV-2 RNA in breathing zone of CW, in room air, with the positivity rate of 2.5% at a concentration of 17 × 103 virus genome copies/m3 air. It also investigates the relationship between local climate conditions [i.e., temperature and relative humidity] and COVID-19 transmission with the evolution of daily official data on the number of new cases, hospitalizations, and deaths. Current data explained that the difference of temperature and humidity may affect the behavior of virus along with other factors, i.e., population density, individual viral shedding, and infectious dose of SARS-CoV-2 (both indoor and outdoor). Our data support the potential SARS-CoV-2 airborne transmission indoors suggesting the specific safety assessment of building to improve ventilation solutions besides proper using face masks and extensive public health interventions.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) revealed in the late 2019 in Wuhan, China, has been the greatest challenges faced by the healthcare system today. The disease called COVID-19 tends to increase the incidence as it overwhelms all attempts at containment and dedicates around, at the time of writing, 182,041,196 infected cases with over 3,698,158 deaths across the world (https://hgis.uw.edu/virus/). Despite repeated infection spread events with different mutations, country after country, the major transmission pathways of SARS-CoV-2 still need to be potentially clarified to better improve infection prevention and control (IPC) policies for healthcare workers (HCWs) and the general public, especially in super-spreading events (Hadei et al., 2020; Lewis, 2020; Noorimotlagh et al., 2021; Ong et al., 2020).
Recent works had been shown that this virus could be transmissible through air and was capable of causing infection for long periods (Guo et al., 2020; Liu et al., 2020; Morawska & Cao, 2020a; Tang et al., 2020). However, there is still no sufficient evidence to compel airborne precautions. In a study by Santarpia et al. (2020), 63.2% of air samples in rooms and 58.3% in hallways of hospital wards treating COVID-19 patients were positive by reverse transcription polymerase chain reaction (RT-PCR) which was more supported by the evidences of virus culture from the hallway sample. The authors concluded that risk of infection was extensive in the vicinity of the patients, none of whom had cough. By using a VIVAS air samplers based on water vapor condensation method, Lednicky et al. (2020a) reaffirmed the occurrence of viable airborne SARS-CoV-2 virus, both at 2 and 4.8 m from patients with viable virus count range between 6 and 74 TCID50 units per liter of air. More importantly, the model of spread of viable SARS-CoV-2 corresponding to a mildly ill with COVID-19 during driving a car with closed windows and air conditioner ON suggested the airborne transmission in vehicles excluding air recirculation in such situations (Lednicky et al., 2021). Therefore, on November 5, 2020, the US CDC accepted the plausibility of airborne particles in propagation of COVID-19 (CDC, 2020). Accordingly, to minimize the airborne transmission of virus, Morawska and Milton (2020b) recommended the measures include (1) providing fresh outdoor air indoors with much higher exchange rates, if possible, and reducing use of air recirculation, (2) infection risk reducing in ventilation systems using germicidal ultraviolet lights and air filtration (HEPA filters), and (3) complete inhibition of crowding in public places. With the supply of entirely negative pressure ventilation and using personal protective equipment (PPE) in COVID-19 ward (CW) locations, lower air sample positivity for SARS-CoV-2 RNA (6%) was observed as opposed to nursing station in the non-COVID-19 ward (17%) where overcrowded by staffs, indicating the congregation of personnel as an important factor in the airborne transmission of the virus in wards not necessarily reserved for COVID-19 patients and inadequately ventilated. The authors stated that there is a lack of data yet on the effectiveness of control approaches of airborne SARS-CoV-2 in prevention of COVID-19 in hospitals (Stern et al., 2021b). Collectively, these studies indicated that the virus can be transmitted as small airborne aerosol particles (< 5 µm) formed during coughing, sneezing, breathing, or talking of an infected and/or susceptible individual with COVID-19, thus enabling its longer travel distance and deep deposition in lungs. Consequently, environmental air sampling gives us substantial evidences to better recognize the airborne transmission and even reemergence risk of virus allowing to stem the chain of future transmission.
In Iran, the study in the largest hospital in March 2020 did not confirm the presence of SARS-CoV-2 RNA in any of the ten air samples taken from various wards of the intensive care units (ICUs) at approximately 2–5 m away from the bed of hospitalized COVID-19 patients (Faridi et al., 2020). They highlighted this knowledge gap whether the transmission of airborne virus is important for the spread of COVID-19. To tackle this question, strong evidence and field experience are needed. Here, we investigated the environmental contamination of air by SARS-CoV-2 in respiratory hospital wards in Zabol, southeastern of Iran.
Material and Methods
Study Site
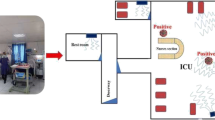
Sina Hospital is the sole COVID-19 patients care center situated in a separate building inside of Amir-al-Momenin Hospital campus in Zabol city, Sistan and Baluchestan province, Iran (31° 2′ N 61° 39′ E). For advanced diagnosis of the disease, firstly patients with involvement in computed tomography (CT) scans were hospitalized to Sina Hospital and then immediately were tested for SARS-CoV-2 RT-PCR assay by the hospital. As the number of hospitalized patients on May 10, 2021, increased, two additional wards: back-up CW1 (BCW1) and back-up CW2 (BCW2), were opened to reduce the density of patients.
Sampling Procedure
Air samples in the ICU, CWs rooms, corridors, nearby nurses’ stations, and toilets (Fig. 1S) were collected as of March 8th through May 12th, 2021. Sampling was performed with midget impingers (SKC Inc., US) which are the most widely used air samplers for collecting aerosolized viable viruses (Pan et al., 2019). For each sample collection, pre-autoclaved impinge was located at 1.5–1.8 m above the ground to represent the breathing zone in the wards and approximately 2–5 m from the patients’ beds within 2 h. As a consequence, a total volume of 180 lit of air was collected using a vacuum pump model (224-PCMTX8, DELUXE, SKC Inc., US) at a flow rate of 1.5 L/min into 5 ml viral transport medium (VTM) per sample. After the 2-h collection time, the remaining volume of the viral medium was about 2 ml due to evaporation which was aseptically transferred to a sterile microtube, sealed with parafilm, and stored at 4 °C prior to immediately transfer to the laboratory (less than 30 min), where samples were immediately stored at − 20 °C for the subsequent analyses. A total of 39 air samples were collected. Each sampling event was started before routine cleaning and disinfection of the areas around 7:30 AM. Indoor air relative humidity (RH, %) and temperature (°C) were measured during sampling by Temperature and Humidity meter GM1361.
SARS-CoV-2 Genomic RNA Detection and Calculation in Sampled Air
In this study, viral RNA was extracted using the Roje Technologies kit (Pishgam, Iran), according to the manufacturer’s instructions. Purified RNA eluted from the RNA-binding silica column in a volume of 60 µl. Real-time quantitative RT-PCR (RT-qPCR) assay was performed using Pishtaz Nucleic Acid Diagnostic kit (Pishtaz Teb, Zaman, Iran) for the nucleocapsid N and RdRp genes of SARS-CoV-2, according to the manufacturer's protocol. 20 µl (final volume) reaction was prepared with 5 µl of purified RNA and 15 µl One-Step RT-qPCR master mix containing a mixture of primers and TaqMan probes for detecting the SARS-CoV-2N and RdRp genes. Amplifications and subsequent analyses were performed by Applied Biosystems Step One plus RT-PCR System. The cycling conditions were reverse transcribed at 50 °C for 20 min, an initial denaturation at 95 °C for 3 min, followed by 45 cycles of 94 °C for 10 s, and 55 °C for 40 s. A sample with cycle threshold (Ct) value of below 40 was regarded as positive. For the Ct values between 40 and 45 cycles, the PCR was repeated. The negative control runs were performed during each experiment, while a positive control template was used for each assay to evaluate the RNA extraction method that had been worked appropriately with 24 and 28 Ct values for RdRp and N genes, respectively. The use of positive control template is essential to confirm the SARS-CoV-2 testing via RT-PCR assay for detecting the adequate performance of nucleic acid extraction, the presence of contamination, and inhibition of the reverse transcription and amplification reactions. Moreover, 2019-nCoV Positive Control (nCoVPC) containing in vitro transcribed RNA was utilized to evaluate the performance of RT-qPCR process in each detection run with 18 and 20 Ct values for RdRp and N genes, respectively. PCR efficiency (E) is reported to be 95% by the respective manufacturer. The limit of detection using this COVID qPCR kit is about 200 copies/ml.
In order to attain the air viral RNA concentration (the number of viral gene copies per m3 air) from the measured Ct values, a 6-log standard curve was used by tenfold dilutions of DNA template of the SARS-CoV-2N and RdRp genes that had been obtained from Pishtaz kit manufacturer. The data were fit using the equation (Eq. 1):
where y = Ct value, GC is genome copies, and (a) and (b) are the slope and the intercept of the regression line, respectively. According to the standard curve, (a) and (b) levels were obtained as follows:
Efficiency % | N gene | RdRp gene | ||||
|---|---|---|---|---|---|---|
Slope, a | Y-intercept, b | R 2 | Slope, a | Y-intercept, b | R 2 | |
95 | 3.6146 | 11.346 | 0.9991 | 3.6175 | 10.96 | 0.9996 |
The gene copy concentration was then obtained from the following steps:
-
(1)
60 μl of total sample RNA purified from 140 μl sample (a 140-µl aliquot was initially taken for nucleic acid extraction) was divided by 5 μl of the purified RNA used in each RT-qPCR to attain the number of gene copies equivalents per 140 μl.
-
(2)
Then, the number of gene copies obtained from Eq. (1) based on the measured Ct levels was multiplied by (60/5) to attain the number of gene copies and finally that number divided by 180 L (the volume of air sampled in 2 h) to attain the number of gene copies per liter of sampled air to report as copies/m3 air.
Statistical Analysis
Multiple linear regression analysis was employed to assess the effect of regional meteorological parameters on the risk of COVID-19 cases during the fourth wave of COVID-19 pandemic in Zabol, with the number of cases as a dependent variable and temperature and relative humidity as independent variables (Luo et al., 2020; Oliveiros et al., 2020; Sajadi et al., 2020). Data analysis was accomplished with IBM SPSS version 22. Statistical significance was considered at P < 0.05. Daily COVID-19 cases’ reports (i.e., new cases, hospitalizations, and deaths) along with temperature and relative humidity data were obtained from the Health Deputy of Zabol University of Medical Sciences and Meteorological Organization in Zabol, respectively, for the period as of February 20 to August 23, 2021.
Results and Discussion
Existence of the SARS-CoV-2 in Different Wards of the Hospital
In the present study, we detected SARS-CoV-2 in air samples (1 of 39, 2.5%) collected from COVID-19 in-patient wards in Zabol (Tables 1, 2). Positive sample was observed on May 11 in the CW Room 15 (a two-bed room housing one patient in bed 16) at a concentration of 17 × 103 copies/m3 air (Fig. S1a). The remaining locations at corridors, patient’s rooms, nursing stations, and toilets in CW, BCW1, BCW2, and ICU did not confirm positive samples. The bed 16 was associated with a patient aged 34 years without cough and was ready to discharge at the time of air sampling collection, indicating the air contamination with SARS-CoV-2 RNA inside patients’ room from a mild COVID-19 patient emanating high viral loads in a poor ventilation conditions.
Positive room air samples related to SARS-CoV-2 RNA have been reported in CWs (Chia et al., 2020; Kenarkoohi et al., 2020; Liu et al., 2020; Santarpia et al., 2020; Stern et al., 2021a). This result contradicts the study from Hong Kong where all air samples were tested negative for SARS-CoV-2, despite the samplers were placed inside a shelter covered around COVID-19 patients at the distance of 10 cm from patients’ chin in order to increase the collection efficiency of exhaled virus and avoid from the effect of environmental air flow (Cheng et al., 2020). Similarly, study in Wuhan No. 7 Hospital also did not report positivity for viral RNA in air samples (Wu et al., 2020).
Patient Behavior
Finding concentration in air samples may be due to the production mechanisms of smaller viral particles (2.5–10 μm) through speaking instead of coughing or desiccation of respiratory droplets (Stern et al., 2021b), which could remain as an airborne infection for 16 h (Fears et al., 2020) and could be carried for long distances. We observed that the patient often was talking on her cell phone during sampling, supporting the hypothesis that SARS-CoV-2 could be shed from a patient via exhaled breath particles which might cause increased environmental risk of airborne transmission (Ma et al., 2021). A great deal of variability in emission rates can be function of source strength. For example, Zhou et al. (2021) indicated that ready-to-discharge patients could shed SARS-CoV-2 RNA up to 7.35–7.77 × 104 viruses per hour during breathing. Buonanno et al. (2020) showed more than 100 quanta emission rates released from an asymptomatic infector in walking mode to fewer than one quanta emission rate from a symptomatic infector in resting mode. The necessity of using a surgical mask by the hospitalized patients is then approved (Cheng et al., 2020).
Air Concentration of SARS-CoV-2
The SARS-CoV-2 concentration in CW (17 × 103 copies/m3) was close to the maximum concentration reported by (Chia et al., 2020; Lednicky et al., 2020a; Ma et al., 2021; Santarpia et al., 2020), but much higher than compared to (Liu et al., 2020; Stern et al., 2021a, 2021b), (< 100 copies/m3) regardless of procedure and volume of air collected (Table 3). The reasons for the discrepancy may in fact be the differences between hospitals. Moreover, as mentioned above due to variability of individual viral shedding, the rate and performance of the ventilation can influence the virus concentration in the air to a low or zero (Morawska et al., 2020). Despite observing high concentration, the positivity rate in our study was lower (2.5%) compared to those in Wuhan (62.8% or 22/35 air samples) (Liu et al., 2020), Kuwait (8.7% or 13/150 air samples) (Stern et al., 2021a), and USA (66.7 or 4/6 air samples) (Lednicky et al., 2020a). It might be because of lower COVID-19 prevalence during sampling event in Zabol between March 8 and May 12, 2021 (before the fourth wave of COVID-19 pandemic) (Fig. 1) and opening the additional CWs (as of May 10, 2021, at the beginning of the fourth wave) to decrease the density of patients. Stern et al. (2021b) concluded that the air positive detected samples correlated positively with the increasing new COVID-19 cases in the community. These results show the early detection of virus in the public can avoid massive infection by identifying pre-symptomatic and asymptomatic individuals through executing the actions such as easily available of testing and quarantine. Also, we did not investigate SARS-CoV-2 spread in other hospital areas (non-CWs) but the existence of SARS-CoV-2 was proved in the air environment where a crowd congregated such as outside the hospital entrances (2 copies m−3) (Stern et al., 2021a), hallway of non-CW (Santarpia et al., 2020), the nurse’s station of non-CWs (about 47 copies m−3) (Stern et al., 2021b), department stores inside of the hospital (11 copies m−3) (Liu et al., 2020), or inside of a car (3.14 × 104 copies m−3) (Lednicky et al., 2021), inside of the Heating, Ventilation, and Air Conditioning (HVAC) systems of dormitories (Sousan et al., 2021), the public places and transportation facilities (Hadei et al., 2021), and at homes of infected individuals (de Man et al., 2021).
Indoor Air Conditions
Ventilation Conditions
None of ICU, BCW1, and BCW2 samples were positive due to the open windows and large amount of dilution by high wind speed (Figs. S1b, S1c and S1d), which highlights the critical role of fresh airflow in removing exhaled virus-bearing air (Ma et al., 2021; Qian et al., 2021). In contrast, work by Guo et al. (2020) explained the different distribution of virus in open cubicles air in the ICU substantiating the abundant presence of SARS-CoV-2 surrounding near-floor exhaust, making it difficult to interpret airborne aerosols. Natural ventilation appeared to be effective at containing airborne SARS-CoV-2 contamination (Tan et al., 2020; Wu et al., 2020) and windows opening are the only way to improve air exchange rates and establishment of cross flows through the building (REHVA, 2020). It was observed that the ventilation conditions in Sina Hospital include negative pressure corridors and toilets through vents installed on the ceiling, while in rooms there was central ceiling-type air conditioning with no exhaust vents in any rooms. Unlike the other wards, more poorly ventilated condition with always all windows closed in CW (for security problems), worst of all in room 15 which had no window, and was weak in ventilation airflow and receiving natural sunlight (Table 1 and Fig. S1a) resulted in a long-range airborne transmission in this room creating increased infection exposure to HCWs contributing to further propagation of the disease. Epidemiologic investigations of super-spreading events indicated that poor ventilation environments led to high number of secondary infections from one super-spreader during the COVID-19 epidemic (Nishiura et al., 2020), especially the super-spreading event in Guangzhou restaurant, in China, was because of airborne transmission where windows could not be opened and ventilation was only performed by available air conditioning systems, with almost the outdoor air ventilation rate of 0.75–1 L/s/person (Li et al., 2020).
Temperature and RH Conditions and Risk of COVID-19
Researchers found that the number of COVID-19 cases correlated with temperature and humidity conditions (Bukhari & Jameel, 2020). Experimental SARS-CoV-2 aerosols are known to remain infectious for 90 min under different environmental conditions, i.e., temperature between 19 and 22 °C and 40–60% of RH (Smither et al., 2020) which is representative of typical closed environments. As shown in Table 1, the SARS-CoV-2 positive sample occurred at the room with condition of RH 41.5% and temperature 24 °C, while at lower RH (11.3–20.2%), samples were all negative. This observation is consistent with previous studies, which have demonstrated that the SARS-CoV-2 virus presence and concentration in air is related to air-affecting factors (Aganovic et al., 2021; Ahlawat et al., 2020; Dabisch et al., 2021) and reported the presence of SARS-CoV-2 at RH condition of 40–50% and temperature of about 20–25 °C (Table 4). Lin and Marr (2019) found that at low RH (≤ 43%), the evaporation rate was high; therefore, viral droplets became excessively dry and formed into smaller particles suspending during long times and migrating farther distances based on ventilation conditions. In contrast, at high RH, the deposition rate was a major factor due to the hygroscopic growth phenomenon causing the droplets to became big and heavy that can be removed from the airflow direction according to their mass. Toward this end, ventilation improvement solutions to best airborne infection control and respiratory health of patients and HCWs in Sina Hospital are mandatory. Furthermore, as ventilation alone cannot remove the risk of infection completely, increasing the cleaning and disinfection (to three times per day) with stricter control of disinfection methods implemented by cleaning crews and use of PPE are in urgent need.
The single positive sample in our study was not sufficient for statistical analysis; therefore, we evaluated the relationship between regional relative humidity (in the range of 9–62%), temperature (in the range of 5.7–41.2 °C), and new COVID-19 cases, hospitalizations, and deaths in Zabol city to associate environmental factors and increased risk of COVID-19 using six months of daily data (as of February 20 to August 23, 2021). Over the association analyses, temperature steadily increased such that an increase of temperature of 1.9 ˚C caused an increase of 0.28 in the risk of new daily cases in Zabol (P < 0.001) (Table 5). Conversely, 0.3 reduction in RH led to 0.2 increase in the risk of COVID-19 cases; however, this association was not statistically significant (P = 0.14). The highest hospitalized cases were related to higher temperature (P < 0.001) (Table 5). These results are in agreement with other studies that concluded that the infection risk (SARS-CoV-2 cases) correlated inversely with humidity (Ahmadi et al., 2020; Gupta et al., 2020; Luo et al., 2020; Ravelli & Martinez, 2021; Sajadi et al., 2020). Similarly, Oliveiros et al., (2020) reported that temperature was correlated positively while humidity was correlated inversely with doubling time of the number of COVID-19 cases in China using linear regression analysis. On the other hand, researchers have demonstrated an inverse relationship between air temperature and number of COVID-19 cases (Kulkarni et al., 2021), suggesting that one standard deviation increase in air temperature would result in a 0.08 lower COVID-19 transmissibility. However, the effect of humidity on the increase of SARS-CoV-2 virus in the outdoor is inconsistent with indoor, this indicates that it may be because of the large contamination attained in the population. On the other words, it is the peak that matters and needs the execution of extensive public health interventions. We also observed the same pattern for the death cases, i.e., higher temperature increased the death cases (P < 0.001), but RH had a negative correlation with deaths (P = 0.047) (Table 6). Current data explained that the difference of temperature and humidity may affect the behavior of virus along with other factors, i.e., population density, individual viral shedding, and infectious dose of SARS-CoV-2 (both indoor and outdoor).
Limitation
Unfortunately, we did not determine the replicability (viability) of the virus identified in the air due to budget limitations and for that reason, the level of inhalation of airborne SARS-CoV-2 to cause infection (infectious dose; TCID50 ml−1) was unclear. Viability should be characterized by tissue culture. Lednicky et al. (2020a) isolated SARS-CoV-2 in room air samples in concentration of 94 × 103 copies m−3 with higher infectious dose of 2.68 × 105 TCID50 ml−1, which estimated 74 viruses require to cause infection in one liter of air. The authors also isolated the viable virus in the smallest particles (0.25–0.5 µm) from inside a car with COVID-19 driver (Lednicky et al., 2021). Moreover, in our study, we were unable to obtain data on air exchanges rates during sampling, and therefore, it was not clear whether the WHO minimum requirements of ventilation rate were met (160 L/s/patient or 12 air changes per hour (ACH) for aerosol generation procedure areas and 60 L/s/patient or 6 ACH for others) (WHO, 2021). In addition, the effect of airflow distribution and direction associated with air exchanges in different regions of the building on air viral load in positive samples as well should be carefully considered. Future studies of identification of wrong airflow direction resulting from poor construction quality would benefit the viral RNA persistence in the respiratory care settings.
Conclusions
The discovery of airborne SARS-CoV-2 expelled into the air of a patient room was further clarified the needs for environmental and engineering monitoring of the respiratory care settings. Our results highlighted the importance of poorly ventilated but not necessarily crowded spaces as a risk factor in persisting SARS-CoV-2 RNA generated by the patients. This may explain the role of indoor air condition as a major risk factor in transmission of SARS‐CoV‐2.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Aganovic, A., Bi, Y., Cao, G., Drangsholt, F., Kurnitski, J., & Wargocki, P. (2021). Estimating the impact of indoor relative humidity on SARS-CoV-2 airborne transmission risk using a new modification of the Wells-Riley model. Building and Environment, 205, 108278.
Ahlawat, A., Wiedensohler, A., & Mishra, S. K. (2020). An overview on the role of relative humidity in airborne transmission of SARS-CoV-2 in indoor environments. Aerosol and Air Quality Research, 20(9), 1856–1861.
Ahmadi, M., Sharifi, A., Dorosti, S., Ghoushchi, S. J., & Ghanbari, N. (2020). Investigation of effective climatology parameters on COVID-19 outbreak in Iran. Science of the Total Environment, 729, 138705.
Baboli, Z., Neisi, N., Babaei, A. A., Ahmadi, M., Sorooshian, A., Birgani, Y. T., & Goudarzi, G. (2021). On the airborne transmission of SARS-CoV-2 and relationship with indoor conditions at a hospital. Atmospheric Environment, 261, 118563.
Bukhari, Q., & Jameel, Y. (2020). Will coronavirus pandemic diminish by summer? Available at SSRN 3556998.
Buonanno, G., Stabile, L., & Morawska, L. (2020). Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environment International, 141, 105794.
CDC, U. (2020). Frequently Asked Questions. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/faq.html#Spread.
Cheng, V.C.-C., Wong, S.-C., Chan, V.W.-M., So, S.Y.-C., Chen, J.H.-K., Yip, C.C.-Y., & Sridhar, S. (2020). Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infection Control & Hospital Epidemiology, 41(11), 1258–1265.
Chia, P. Y., Coleman, K. K., Tan, Y. K., Ong, S. W. X., Gum, M., Lau, S. K., & Lee, P. H. (2020). Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature Communications, 11(1), 1–7.
Dabisch, P., Schuit, M., Herzog, A., Beck, K., Wood, S., Krause, M., & Hooper, I. (2021). The influence of temperature, humidity, and simulated sunlight on the infectivity of SARS-CoV-2 in aerosols. Aerosol Science and Technology, 55(2), 142–153.
de Man, P., Ortiz, M. A., Bluyssen, P. M., de Man, S. J., Rentmeester, M.-J., van der Vliet, M., & Ong, D. S. (2022). Airborne SARS-CoV-2 in home and hospital environments investigated with a high-powered air sampler. Journal of Hospital Infection., 119, 126–131.
Faridi, S., Niazi, S., Sadeghi, K., Naddafi, K., Yavarian, J., Shamsipour, M., & Yunesian, M. (2020). A field indoor air measurement of SARS-CoV-2 in the patient rooms of the largest hospital in Iran. Science of the Total Environment, 725, 138401.
Fears, A. C., Klimstra, W. B., Duprex, P., Hartman, A., Weaver, S. C., Plante, K. S., & Fernández, D. (2020). Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerging Infectious Diseases, 26(9), 2168.
Guo, Z.-D., Wang, Z.-Y., Zhang, S.-F., Li, X., Li, L., Li, C., & Chi, X.-Y. (2020). Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging Infectious Diseases, 26(7), 1586.
Gupta, S., Raghuwanshi, G. S., & Chanda, A. (2020). Effect of weather on COVID-19 spread in the US: A prediction model for India in 2020. Science of the Total Environment, 728, 138860.
Hadei, M., Hopke, P. K., Jonidi, A., & Shahsavani, A. (2020). A letter about the airborne transmission of SARS-CoV-2 based on the current evidence. Aerosol and Air Quality Research, 20(5), 911–914.
Hadei, M., Mohebbi, S. R., Hopke, P. K., Shahsavani, A., Bazzazpour, S., Alipour, M., & Yarahmadi, M. (2021). Presence of SARS-CoV-2 in the air of public places and transportation. Atmospheric Pollution Research, 12(3), 302–306.
Kenarkoohi, A., Noorimotlagh, Z., Falahi, S., Amarloei, A., Mirzaee, S. A., Pakzad, I., & Bastani, E. (2020). Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. Science of the Total Environment, 748, 141324.
Kulkarni, H., Khandait, H., Narlawar, U. W., Rathod, P., & Mamtani, M. (2021). Independent association of meteorological characteristics with initial spread of Covid-19 in India. Science of the Total Environment, 764, 142801.
Lednicky, J. A., Lauzard, M., Fan, Z. H., Jutla, A., Tilly, T. B., Gangwar, M., & Eiguren-Fernandez, A. (2020a). Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. International Journal of Infectious Diseases, 100, 476–482.
Lednicky, J. A., Shankar, S. N., Elbadry, M. A., Gibson, J. C., Alam, M. M., Stephenson, C. J., & Salemi, M. (2020b). Collection of SARS-CoV-2 virus from the air of a clinic within a university student health care center and analyses of the viral genomic sequence. Aerosol and Air Quality Research, 20(6), 1167.
Lednicky, J. A., Lauzardo, M., Alam, M. M., Elbadry, M. A., Stephenson, C. J., Gibson, J. C., & Morris, J. G., Jr. (2021). Isolation of SARS-CoV-2 from the air in a car driven by a COVID patient with mild illness. International Journal of Infectious Diseases., 108, 212–216.
Lewis, D. (2020). Is the coronavirus airborne? Experts can’t agree. Nature, 580(7802), 175.
Li, Y., Qian, H., Hang, J., Chen, X., Hong, L., Liang, P., & Liu, L. (2020). Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. MedRxiv.
Lin, K., & Marr, L. C. (2019). Humidity-dependent decay of viruses, but not bacteria, in aerosols and droplets follows disinfection kinetics. Environmental Science & Technology, 54(2), 1024–1032.
Liu, Y., Ning, Z., Chen, Y., Guo, M., Liu, Y., Gali, N. K., & Westerdahl, D. (2020). Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature, 582(7813), 557–560.
Luo, W., Majumder, M., Liu, D., Poirier, C., Mandl, K., Lipsitch, M., & Santillana, M. (2020). The role of absolute humidity on transmission rates of the COVID-19 outbreak.
Ma, J., Qi, X., Chen, H., Li, X., Zhang, Z., Wang, H., & Morawska, L. (2021). Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 per hour. Clinical Infectious Diseases, 72(10), e652–e654.
Morawska, L., & Cao, J. (2020a). Airborne transmission of SARS-CoV-2: The world should face the reality. Environment International, 139, 105730.
Morawska, L., & Milton, D. K. (2020b). It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clinical Infectious Diseases, 71(9), 2311–2313.
Morawska, L., Tang, J. W., Bahnfleth, W., Bluyssen, P. M., Boerstra, A., Buonanno, G., & Franchimon, F. (2020). How can airborne transmission of COVID-19 indoors be minimised? Environment International, 142, 105832.
Nishiura, H., Oshitani, H., Kobayashi, T., Saito, T., Sunagawa, T., Matsui, T., & Suzuki, M. (2020). Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). MedRxiv.
Noorimotlagh, Z., Mirzaee, S. A., Kalantar, M., Barati, B., Fard, M. E., & Fard, N. K. (2021). The SARS-CoV-2 (COVID-19) pandemic in hospital: An insight into environmental surfaces contamination, disinfectants’ efficiency, and estimation of plastic waste production. Environmental Research, 202, 111809.
Oliveiros, B., Caramelo, L., Ferreira, N. C., & Caramelo, F. (2020). Role of temperature and humidity in the modulation of the doubling time of COVID-19 cases. MedRxiv.
Ong, S. W. X., Tan, Y. K., Chia, P. Y., Lee, T. H., Ng, O. T., Wong, M. S. Y., & Marimuthu, K. (2020). Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA, 323(16), 1610–1612.
Pan, M., Lednicky, J. A., & Wu, C. Y. (2019). Collection, particle sizing and detection of airborne viruses. Journal of Applied Microbiology, 127(6), 1596–1611.
Qian, H., Miao, T., Liu, L., Zheng, X., Luo, D., & Li, Y. (2021). Indoor transmission of SARS-CoV-2. Indoor Air, 31(3), 639–645.
Ravelli, E., & Martinez, R. G. (2021). Environmental risk factors of airborne viral transmission: Humidity, influenza and SARS-CoV-2 in the Netherlands. Spatial and Spatio-temporal Epidemiology, 100432.
REHVA. (2020). COVID-19 guidance document.
Sajadi, M. M., Habibzadeh, P., Vintzileos, A., Shokouhi, S., Miralles-Wilhelm, F., & Amoroso, A. (2020). Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of coronavirus disease 2019 (COVID-19). JAMA Network Open, 3(6), e2011834–e2011834.
Santarpia, J. L., Rivera, D. N., Herrera, V. L., Morwitzer, M. J., Creager, H. M., Santarpia, G. W., & Broadhurst, M. J. (2020). Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Scientific Reports, 10(1), 1–8.
Smither, S. J., Eastaugh, L. S., Findlay, J. S., & Lever, M. S. (2020). Experimental aerosol survival of SARS-CoV-2 in artificial saliva and tissue culture media at medium and high humidity. Emerging Microbes & Infections, 9(1), 1415–1417.
Sousan, S., Fan, M., Outlaw, K., Williams, S., & Roper, R. L. (2021). SARS-CoV-2 detection in air samples from inside heating, ventilation, and air conditioning (HVAC) systems-COVID surveillance in student dorms. American Journal of Infection Control.
Stern, R. A., Al-Hemoud, A., Alahmad, B., & Koutrakis, P. (2021a). Levels and particle size distribution of airborne SARS-CoV-2 at a healthcare facility in Kuwait. Science of the Total Environment, 782, 146799.
Stern, R. A., Koutrakis, P., Martins, M. A., Lemos, B., Dowd, S. E., Sunderland, E. M., & Garshick, E. (2021b). Characterization of hospital airborne SARS-CoV-2. Respiratory Research, 22(1), 1–8.
Tan, L., Ma, B., Lai, X., Han, L., Cao, P., Zhang, J., & Wang, Z. (2020). Air and surface contamination by SARS-CoV-2 virus in a tertiary hospital in Wuhan, China. International Journal of Infectious Diseases, 99, 3–7.
Tang, S., Mao, Y., Jones, R. M., Tan, Q., Ji, J. S., Li, N., & Ding, P. (2020). Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environment International, 144, 106039.
WHO. (2021). Roadmap to improve and ensure good indoor ventilation in the context of COVID-19. WHO.
Wu, S., Wang, Y., Jin, X., Tian, J., Liu, J., & Mao, Y. (2020). Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. American Journal of Infection Control, 48(8), 910–914.
Zhou, L., Yao, M., Zhang, X., Hu, B., Li, X., Chen, H., & Sun, B. (2021). Breath-, air-and surface-borne SARS-CoV-2 in hospitals. Journal of Aerosol Science, 152, 105693.
Acknowledgements
The authors would like to thank the staff of Sina Hospital and also Imam Khomeini Hospital for their assistance in sampling period. We gratefully acknowledge the Health Deputy of Zabol University of Medical Sciences and Meteorological Organization for providing daily COVID-19 cases’ reports and meteorology data. This work was financially supported by Zabol University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
AZ contributed to conceptualization, methodology, formal analysis, writing—editing draft, and investigation. FS contributed to methodology, investigation, and project administration. MG contributed to formal analysis, writing—original draft, conceptualization, methodology, investigation, and resources. AK contributed to project administration, investigation, validation, and conceptualization. MRRK contributed to project administration, investigation, resources, and data curation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zahedi, A., Seif, F., Golshan, M. et al. Air Surveillance for Viral Contamination with SARS-CoV-2 RNA at a Healthcare Facility. Food Environ Virol 14, 374–383 (2022). https://doi.org/10.1007/s12560-022-09524-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-022-09524-1