Abstract
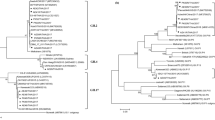
Norovirus (NoV) is a major cause of non-bacterial acute gastroenteritis worldwide, and the variants of genotype GII.4 are currently the predominant human strains. Recently, a novel variant of NoV GII.17 (GII.P17_GII.17 NoV), termed Kawasaki 2014, has been reported as the cause of gastroenteritis outbreaks in Asia, replacing the pandemic strain GII.4 Sydney 2012. The GII.17 Kawasaki 2014 variant has also been reported sporadically in patients with gastroenteritis outside of Asia, including Italy. In this study, 384 shellfish samples were subjected to screening for human NoVs using real-time PCR and 259 (67.4%) tested positive for Genogroup II (GII) NoV. Of these, 52 samples, selected as representative of different areas and sampling dates, were further amplified by conventional PCR targeting the capsid gene, using broad-range primers. Forty shellfish samples were characterized by amplicon sequencing as GII.4 (n = 29), GII.2 (n = 4), GII.6 (n = 2), GII.12 (n = 2), and GII.17 (n = 3). Sixty-eight water samples (39 seawater samples from the corresponding shellfish production areas and 29 water samples from nearby underwater sewage discharge points) were also tested using the above broad-range assay: eight NoV-positive samples were characterized as GII.1 (n = 3), GII.2 (n = 1), GII.4 (n = 2), and GII.6 (n = 2). Based on full genome sequences available in public databases, a novel RT-PCR nested assay specific for GII.17 NoVs was designed and used to re-test the characterized shellfish (40) and water (8) samples. In this second screening, the RNA of GII.17 NoV was identified in 17 additional shellfish samples and in one water sample. Upon phylogenetic analysis, these GII.17 NoV isolates were closely related to the novel GII.17 Kawasaki 2014. Interestingly, our findings chronologically matched the emergence of the Kawasaki 2014 variant in the Italian population (early 2015), as reported by hospital-based NoV surveillance. These results, showing GII.17 NoV strains to be widespread in shellfish samples collected in 2015 in Italy, provide indirect evidence that this strain has started circulating in the Italian population. Notably, using a specific assay, we were able to detect many more samples positive for GII.17 NoV, indicating that, in food and water matrices, broad-range assays for NoV may grossly underestimate the prevalence of some, less common, NoVs. The detection of the GII.17 strain Kawasaki 2014 in clinical, water and food samples in Italy highlights the need for more systematic surveillance for future disease control and prevention.


Similar content being viewed by others
References
Chan, M. C., Lee, N., Hung, T. N., Kwok, K., Cheung, K., Tin, E. K., et al. (2015). Rapid emergence and predominance of a broadly recognizing and fast-evolving Norovirus GII.17 variant in late, 2014. Nature Communications, 6, 10061.
Chen, H., Qian, F., Xu, J., Chan, M., Shen, Z., Zai, S., et al. (2015). A novel Norovirus GII.17 lineage contributed to adult gastroenteritis in Shanghai, China, during the winter of 2017-2015. Emerging Microbes & Infections, 4, e67.
Costafreda, M. I., Bosch, A., & Pintó, R. M. (2006). Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Applied and Environmental Microbiology, 72(6), 3846–3855.
da Silva, A. K., le Saux, J. C., Parnaudeau, S., Pommepuy, M., Elimelech, M., & le Guyader, F. S. (2007). Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: Different behaviors of genogroups I and II. Applied and Environmental Microbiology, 73(24), 7891–7897.
de Graaf, M., van Beek, J., Vennema, H., Podkolzin, A. T., Hewitt, J., Bucardo, F., et al. (2015). Emergence of a novel GII.17 norovirus - End of the GII.4 era? Euro Surveillance, 20(26), 1–8.
Dinu, S., Nagy, M., Negru, D. G., Popovici, E. D., Zota, L., & Oprisan, G. (2016). Molecular identification of emergent GII.P17-GII.17 norovirus genotype, Romania, 2015. Euro Surveillance, 21, 30141.
Green, K. Y. (2013). Caliciviridae: The noroviruses. In D. M. Knipe & P. M. Howley (Eds.), Fields virology (6th ed., pp. 949–979). Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins.
Iaconelli, M., Muscillo, M., Della Libera, S., Fratini, M., Meucci, L., De Ceglia, M., et al. (2017). One-year surveillance of human enteric viruses in raw and treated wastewaters, downstream river waters, and drinking waters. Food and Environmental Virology, 9(1), 79–88. doi:10.1007/s12560-016-9263-3.
Iaconelli, M., Purpari, G., Della Libera, S., Petricca, S., Guercio, A., Ciccaglione, A. R., et al. (2015). Hepatitis A and E viruses in wastewaters, in river waters, and in Bivalve Molluscs in Italy. Food and Environmental Virology, 7(4), 316–324. doi:10.1007/s12560-015-9207-3.
Imamura, S., Haruna, M., Goshima, T., Kanezashi, H., Okada, T., & Akimoto, K. (2016). Application of next-generation sequencing to investigation of norovirus diversity in shellfish collected from two coastal sites in Japan from 2013 to 2014. Japanese Journal of Veterinary Research, 64(2), 113–122.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., et al. (2003). Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41(4), 1548–1557.
Kazama, S., Masago, Y., Tohma, K., Souma, N., Imagawa, T., Suzuki, A., et al. (2016). Temporal dynamics of norovirus determined through monitoring of municipal wastewater by pyrosequencing and virological surveillance of gastroenteritis cases. Water Research., 92, 244–253.
Khamrin, P., Kumthip, K., Yodmeeklin, A., Supadej, K., Ukarapol, N., Thongprachum, A., et al. (2016). Molecular characterization of norovirus GII.17 detected in healthy adult, intussusception patient, and acute gastroenteritis children in Thailand. Infection, Genetics and Evolution, 25(44), 330–333.
Kiulia, N. M., Mans, J., Mwenda, J. M., & Taylor, M. B. (2014). Norovirus GII.17 predominates in selected surface water sources in Kenya. Food and Environmental Virology, 6(4), 221–231.
Kojima, S., Kageyama, T., Fukushi, S., Hoshino, F. B., Shinohara, M., Uchida, K., et al. (2002). Genogroup-specific PCR primers for detection of Norwalk-like viruses. Journal of Virology, 100(1–2), 107–114.
Kroneman, A., Vega, E., Vennema, H., Vinjé, J., White, P. A., Hansman, G., et al. (2013). Proposal for a unified norovirus nomenclature and genotyping. Archives of Virology, 158(10), 2059–2068.
Kroneman, A., Vennema, H., Deforche, K., Avoort, H. V. D., Peñaranda, S., Oberste, M. S., et al. (2011). An automated genotyping tool for enteroviruses and noroviruses. Journal of Clinical Virology, 51(2), 121–125.
Le Blanc, J. J., Pettipas, J., Gaston, D., Taylor, R., Hatchette, T. F., Booth, T. F., et al. (2016). Outbreak of Norovirus GII.P17-GII.17 in the Canadian Province of Nova Scotia. Canadian Journal of Infectious Diseases and Medical Microbiology, 2016, 1280247.
Lee, C. C., Feng, Y., Chen, S. Y., Tsai, C. N., Lai, M. W., & Chiu, C. H. (2015). Emerging norovirus GII.17 in Taiwan. Clinical Infectious Diseases, 61, 1762–1764.
Loisy, F., Atmar, R. L., Guillon, P., Le Cann, P., Pommepuy, M., & Le Guyader, F. S. (2005). Real-time RT-PCR for norovirus screening in shellfish. Journal of Virological Methods, 123(1), 1–7.
Lopman, B., Vennema, H., Kohli, E., Pothier, P., Sanchez, A., Negredo, A., et al. (2004). Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet, 363, 682–688.
Lu, J., Fang, L., Zheng, H., Lao, J., Yang, F., Sun, L., et al. (2016). The Evolution and Transmission of Epidemic GII.17 Noroviruses. Journal of Infectious Diseases, 214, 556–564.
Lu, J., Sun, L., Fang, L., Yang, F., Mo, Y., Lao, J., et al. (2015). Gastroenteritis outbreaks caused by norovirus GII.17, Guangdong Province, China, 2014-2015. Emerging Infectious Disease, 21, 1240–1242.
Maletskaya, O. V., Tibilov, A. G., Prislegina, D. A., Gazieva, G. K., Otaraeva, N. I., Volynkina, A. S., et al. (2016). Epidemiologic features of norovirus infection outbreak in the Republic of North Ossetia-Alania. Zh Mikrobiol Epidemiol Immunobiol, 2, 69–74.
Matsushima, Y., Ishikawa, M., Shimizu, T., Komane, A., Kasuo, S., Shinohara, M., et al. (2015). Genetic analyses of GII.17 norovirus strains in diarrheal disease outbreaks from December 2014 to March 2015 in Japan reveal a novel polymerase sequence and amino acid substitutions in the capsid region. Euro Surveillance, 20(26), 21173.
Medici, M. C., Tummolo, F., Calderaro, A., Chironna, M., Giammanco, G. M., De Grazia, S., et al. (2015). Identification of the novel Kawasaki 2014 GII. 17 human norovirus strain in Italy. Eurosurveillance, 20(35), 30010.
Muscillo, M., Fratini, M., Graffeo, R., Sanguinetti, M., Martella, V., Green, K. Y., et al. (2013). GIV noroviruses in wastewaters and in stool specimens from hospitalized patients. Food and Environmental Virology, 5(4), 194–202.
Parra, G. I., & Green, K. Y. (2015). Genome of emerging norovirus GII.17, United States, 2014. Emerging Infectious Diseases, 21, 1477–1479.
Prevost, B., Lucas, F. S., Ambert-Balay, K., Pothier, P., Moulin, L., & Wurtzer, S. (2015). Deciphering the diversities of astroviruses and noroviruses in wastewater treatment plant effluents by a high-throughput sequencing method. Applied and Environmental Microbiology, 81, 7215–7222.
Pu, J., Kazama, S., Miura, T., Azraini, N. D., Konta, Y., Ito, H., et al. (2016). Pyrosequencing analysis of norovirus genogroup II distribution in sewage and oysters: First detection of GII.17 Kawasaki 2014 in Oysters. Food and Environmental Virology, 8(4), 310–312.
Qin, M., Dong, X. G., Jing, Y. Y., Wei, X. X., Wang, Z. E., Feng, H. R., et al. (2016). A waterborne gastroenteritis outbreak caused by norovirus GII.17 in a Hotel, Hebei, China, December 2014. Food and Environmental Virology, 8(3), 180–186.
Rajko-Nenow, P., Waters, A., Keaveney, S., Flannery, J., Tuite, G., Coughlan, S., et al. (2013). Norovirus genotypes present in oysters and in effluent from a wastewater treatment plant during the seasonal peak of infections in Ireland in 2010. Applied and Environmental Microbiology, 79(8), 2578–2587.
Rasmussen, L. D., Schultz, A. C., Uhrbrand, K., Jensen, T., & Fischer, T. K. (2016). Molecular evidence of oysters as vehicle of norovirus GII.P17-GII.17. Emerging Infectious Diseases, 22(11), 2024–2025.
Schorn, R., Hohne, M., Meerbach, A., Bossart, W., Wuthrich, R. P., Schreier, E., et al. (2010). Chronic norovirus infection after kidney transplantation: molecular evidence for immune-driven viral evolution. Clinical Infectious Diseases, 51, 307–314.
Siebenga, J. J., Lemey, P., Kosakovsky Pond, S. L., Rambaut, A., Vennema, H., & Koopmans, M. (2010). Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathogens, 6, e1000884.
Siebenga, J. J., Vennema, H., Renckens, B., de Bruin, E., van der Veer, B., Siezen, R. J., & Koopmans, M. (2007). Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. Journal of Virology, 81(18), 9932–9941.
Singh, B. K., Leuthold, M. M., & Hansman, G. S. (2015). Human noroviruses’ fondness for histo-blood group antigens. Journal of Virology, 89(4), 2024–2040.
Sukhrie, F. H., Beersma, M. F., Wong, A., van der Veer, B., Vennema, H., Bogerman, J., et al. (2011). Using molecular epidemiology to trace transmission of nosocomial norovirus infection. Journal of Clinical Microbiology, 49, 602–606.
Svraka, S., Duizer, E., Vennema, H., de Bruin, E., van der Veer, B., Dorresteijn, B., et al. (2007). Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. Journal of Clinical Microbiology, 45(5), 1389–1394.
Tran, T. H., Trainor, E., Nakagomi, T., Cunliffe, N. A., & Nakagomi, O. (2013). Molecular epidemiology of noroviruses associated with acute sporadic gastroenteritis in children: global distribution of genogroups, genotypes and GII.4 variants. Journal of Clinical Virology, 56(3), 269–277.
van Beek, J., Ambert-Balay, K., Botteldoorn, N., Eden, J. S., Fonager, J., Hewitt, J., et al. (2013). Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveillance, 18, 8–9.
Victoria, M., Tort, L. F., Lizasoain, A., Garcia, M., Castells, M., Berois, M., et al. (2016). Norovirus molecular detection in Uruguayan sewage samples reveals a high genetic diversity and GII.4 variant replacement along time. Journal of Applied Microbiology, 120(5), 1427–1435.
Vinje, J. (2015). Advances in laboratory methods for detection and typing of norovirus. Journal of Clinical Microbiology, 53, 373–381.
Zhou, N., Lin, X., Wang, S., Tao, Z., Xiong, P., Wang, H., et al. (2016). Molecular epidemiology of GI and GII noroviruses in sewage: 1-year surveillance in eastern China. Journal of Applied Microbiology, 121(4), 1172–1179.
Acknowledgements
This research has been partially founded by the Italian Ministry of Health, Finalized Research Project “Vibrio and viruses in shellfish: old and emerging pathogens. Evaluation of exposure levels for the implementation of prevention strategies” RF-2011-02349693. We thank Professor Herbert W. Virgin, Washington University (St. Louis, Missouri, United States) for providing the murine Norovirus strain used as sample process control.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
La Rosa, G., Della Libera, S., Iaconelli, M. et al. Detection of Norovirus GII.17 Kawasaki 2014 in Shellfish, Marine Water and Underwater Sewage Discharges in Italy. Food Environ Virol 9, 326–333 (2017). https://doi.org/10.1007/s12560-017-9290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-017-9290-8