Abstract
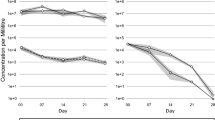
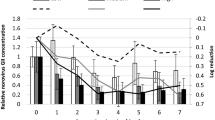
This study characterizes the persistence of human norovirus in Eastern oysters (Crassostrea virginica) held at different seawater temperatures. Oysters were contaminated with human norovirus GI.1 (Norwalk strain 8FIIa) by exposing them to virus-contaminated water at 15 °C, and subsequently holding them at 7, 15, and 25 °C for up to 6 weeks. Viral RNA was extracted from oyster tissue and hemocytes and quantitated by RT-qPCR. Norovirus was detected in hemocytes and oysters held at 7 and 15 °C for 6 weeks and in hemocytes and oysters held at 25 °C for up to 2 and 4 weeks, respectively. Results confirm that NoV is quite persistent within oysters and demonstrate that cooler water temperatures extend norovirus clearance times. This study suggests a need for substantial relay times to remove norovirus from contaminated shellfish and suggests that regulatory authorities should consider the effects of water temperature after a suspected episodic norovirus-contamination event.


Similar content being viewed by others
References
Ang, L. H. (1998). An outbreak of viral gastroenteritis associated with eating raw oysters. Communicable Disease and Public Health, 1, 38–40.
Burkhardt, W, 3rd, & Calci, K. R. (2000). Selective accumulation may account for shellfish-associated viral illness. Applied and Environmental Microbiology, 66, 1375–1378.
Dancho, B. A., Chen, H., & Kingsley, D. H. (2012). Discrimination between infectious and non-infectious human norovirus using porcine gastric mucin. International Journal of Food Microbiology, 155, 222–226.
DePaola, A., Jones, J. L., Woods, J., Burkhardt, W, 3rd, Calci, K. R., Krantz, J. A., et al. (2010). Bacterial and viral pathogens in live oysters: 2007 United States market survey. Applied and Environmental Microbiology, 76, 2754–2768.
Grodzki, M., Ollivier, J., Le Saux, J. C., Piquet, J. C., Noyer, M., & Le Guyader, F. S. (2012). Impact of xynthia tempest on viral contamination of shellfish. Applied and Environmental Microbiology, 78, 3508–3511.
Grohmann, G. S., Murphy, A. M., Christopher, P. J., Auty, E., & Greenberg, H. B. (1981). Norwalk virus gastroenteritis in volunteers consuming depurated oysters. The Australian Journal of Experimental Biology and Medical Science, 59, 219–228.
Iwamoto, M., Ayers, T., Mahon, B. E., & Swerdlow, D. L. (2010). Epidemiology of seafoodassociated infections in the United States. Clinical Microbiology Reviews, 23, 399–411.
Kageyama, T., Kojima, S., Shinohara, M., Uchida, K., Fukushi, S., Hoshino, F. B., et al. (2003). Broadly reactive and highly sensitive assay for norwalk-like viruses based on real-time quantitative reverse transcription-PCR. Journal of Clinical Microbiology, 41, 1548–1557.
Kingsley, D. H., & Richards, G. P. (2001). Rapid and efficient extraction method for reverse transcription-PCR detection of hepatitis A and norwalk-like viruses in shellfish. Applied and Environmental Microbiology, 67, 4152–4157.
Langlet, J., Kaas, L., & Greening, G. (2015). Binding-based RT-qPCR assay to assess binding patterns of noroviruses to shellfish. Food and environmental virology, 7(2), 88–95.
Le Guyader, F. S., Neill, F. H., Dubois, E., Bon, F., Loisy, F., Kohli, E., et al. (2003). A semiquantitative approach to estimate Norwalk-like virus contamination of oysters implicated in an outbreak. International Journal of Food Microbiology, 87, 107–112.
Lowther, J. A., Gustar, N. E., Hartnell, R. E., & Lees, D. N. (2012a). Comparison of norovirus RNA levels in outbreak-related oysters with background environmental levels. Journal of Food Protection, 75, 389–393.
Lowther, J. A., Gustar, N. E., Powell, A. L., Hartnell, R. E., & Lees, D. N. (2012b). Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the united kingdom. Applied and Environmental Microbiology, 78, 5812–5817.
Maalouf, H., Zakhour, M., Le Pendu, J., Le Saux, J. C., Atmar, R. L., & Le Guyader, F. S. (2010). Distribution in tissue and seasonal variation of norovirus genogroup I and II ligands in oysters. Applied and Environmental Microbiology, 76, 5621–5630.
Nappier, S. P., Graczyk, T. K., & Schwab, K. J. (2008). Bioaccumulation, retention, and depuration of enteric viruses by crassostrea virginica and crassostrea ariakensis oysters. Applied and Environmental Microbiology, 74, 6825–6831.
Neish, A. (2013). Investigative trials on the purification of oysters to identify ways of reducing norovirus. CEFAS Contract Report c5224.
Nishida, T., Nishio, O., Kato, M., Chuma, T., Kato, H., Iwata, H., et al. (2007). Genotyping and quantitation of noroviruses in oysters from two distinct sea areas in japan. Microbiology and Immunology, 51, 177–184.
Praveen, C., Dancho, B. A., Kingsley, D. H., Calci, K. R., Meade, G. K., Mena, K. D., & Pillai, S. D. (2013). Susceptibility of murine norovirus and hepatitis A virus to electron beam irradiation in oysters and quantifying the reduction in potential infection risks. Applied and Environmental Microbiology, 79, 3796–3801.
Provost, K., Dancho, B. A., Ozbay, G., Anderson, R. S., Richards, G. P., & Kingsley, D. H. (2011). Hemocytes are sites of enteric virus persistence within oysters. Applied and Environmental Microbiology, 77, 8360–8369.
Richards, G. P. (1988). Microbial purification of shellfish: A review of depuration and relaying. Journal of Food Protection, 51, 218–251.
Richards, G. P., Watson, M. A., & Kingsley, D. H. (2004). A SYBR green real-time RT-PCR method to detect and quantitate Norwalk virus in stools. Journal of Virological Methods, 116, 63–70.
Rodriguez-Manzano, J., Hundesa, A., Calgua, B., Carratala, A., Maluquer de Motes, C., Rusinol, M., et al. (2013). Adenovirus and norovirus contaminants in commercially distributed shellfish. Food and Environmental Virology, 6, 31–41.
Rohayem, J. (2009). Norovirus seasonality and the potential impact of climate change. Clinical Microbiology & Infection, 15, 524–527.
Savini, G., Casaccia, C., Barile, N. B., Paoletti, M., & Pinoni, C. (2009). Norovirus in bivalve molluscs: A study of the efficacy of the depuration system. Veterinaria Italiana, 45, 535–539.
Schaeffer, J., Le Saux, J. C., Lora, M., Atmar, R. L., & Le Guyader, F. S. (2013). Norovirus contamination on French marketed oysters. International Journal of Food Microbiology, 166, 244–248.
Shumway, S. E. (1996). The Eastern oyster: Crassostrea virginica. In V. S. Kennedy, R. I. E. Newell, & A. F. Eble (Eds.), Natural environmental factors (pp. 467–513). College Park: the Maryland Sea Grant College.
Tian, P., Bates, A. H., Jensen, H. M., & Mandrell, R. E. (2006). Norovirus binds to blood group A-like antigens in oyster gastrointestinal cells. Letters in Applied Microbiology, 43, 645–651.
Tian, P., Engelbrektson, A. L., Jiang, X., Zhong, W., & Mandrell, R. E. (2007). Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: A possible mechanism of bioaccumulation. Journal of Food Protection, 70, 2140–2147.
Ueki, Y., Shoji, M., Suto, A., Tanabe, T., Okimura, Y., Kikuchi, Y., et al. (2007). Persistence of caliciviruses in artificially contaminated oysters during depuration. Applied and Environmental Microbiology, 73, 5698–5701.
Acknowledgments
We wish to thank Gary Richards and Shiowshuh Sheen for critical reading of the manuscript. This work was made possible by intramural funding for the US Department of Agriculture, Agricultural Research Service and a grant from the National Research Foundation of Korea (NRF-2013R1A1A1064168). The US Department of Agriculture is an equal opportunity provider and employer. Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply a recommendation or endorsement by the US Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, C., Kingsley, D.H. Temperature-Dependent Persistence of Human Norovirus Within Oysters (Crassostrea virginica). Food Environ Virol 8, 141–147 (2016). https://doi.org/10.1007/s12560-016-9234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-016-9234-8