Abstract
The subjectivity and inaccuracy of in-clinic Cognitive Health Assessments (CHA) have led many researchers to explore ways to automate the process to make it more objective and to facilitate the needs of the healthcare industry. Artificial Intelligence (AI) and machine learning (ML) have emerged as the most promising approaches to automate the CHA process. In this paper, we explore the background of CHA and delve into the extensive research recently undertaken in this domain to provide a comprehensive survey of the state-of-the-art. In particular, a careful selection of significant works published in the literature is reviewed to elaborate a range of enabling technologies and AI/ML techniques used for CHA, including conventional supervised and unsupervised machine learning, deep learning, reinforcement learning, natural language processing, and image processing techniques. Furthermore, we provide an overview of various means of data acquisition and the benchmark datasets. Finally, we discuss open issues and challenges in using AI and ML for CHA along with some possible solutions. In summary, this paper presents CHA tools, lists various data acquisition methods for CHA, provides technological advancements, presents the usage of AI for CHA, and open issues, challenges in the CHA domain. We hope this first-of-its-kind survey paper will significantly contribute to identifying research gaps in the complex and rapidly evolving interdisciplinary mental health field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive health assessments (CHA) measure an individual’s cognitive abilities and functioning. These assessments are typically used in diagnosing and monitoring conditions that affect cognitive abilities, such as dementia, Alzheimer’s disease, and other forms of cognitive decline. They can also assess cognitive abilities in individuals with brain injuries and neurological conditions. CHA typically involves a series of tests and tasks designed to evaluate specific aspects of cognitive function, such as memory, attention, language, reasoning, and perception. Healthcare professionals, such as neurologists, psychologists, or occupational therapists, usually administer these assessments. The dysfunction or inability of the brain to perform cognitive functions such as learning, thinking, remembering, comprehension, decision-making, and attention has been termed Cognitive Impairment (CI) [1]. A natural cognitive decline occurs as one ages; however, if this decline is more than what usually comes with age, it is termed the onset of Mild Cognitive Impairment (MCI) [2]. MCI can be triggered by stress, depression, stroke, brain injury, and other underlying health conditions. The condition gradually progresses from Early Mild Cognitive Impairment (EMCI) to MCI to Late Mild Cognitive Impairment (LMCI), and finally, to Alzheimer’s Disease (AD). Although AD is the most frequent type of dementia, MCI could progress to dementia with Lewy bodies as well. The impairment in all these stages affects a patient’s daily activities and can show varying degrees of cognitive decline symptoms [3, 4]. Figure 1 presents the early signs of MCI.
According to the Alzheimer’s Association, a leading voluntary health organisation in Alzheimer’s care, support, and research, by 2050, the number of AD patients is expected to rise to 13.8 million. The overall cost of care and management of AD is very high, and in 2019 alone, it was valued at $290 billion. Hence, it has been observed that diagnosed and managed MCI provides medical benefits and reduces long-term care costs. Forecasts suggest that early diagnosis in the MCI stage in the year 2050 can bring health care costs down by $231 billion [5].
MCI screening and early intervention is currently the most widely accepted strategy to manage AD. Diagnosis of MCI is established through various assessments. These assessments not only help diagnose but also help characterise cognition at the stage of MCI; hence they may help in understanding the cognitive pathophysiology [6]. Unfortunately, the lack of proper guidelines and standardised screening has resulted in undiagnosed MCI. The likelihood of this disease's progression and decline in cognitive health is becoming common. With the advent of the Internet of Medical Things (IoMT) and Artificial Intelligence (AI), several researchers have proposed automated assessment techniques to improve the accuracy of diagnosis. Needless to say, this has been driven by AI and Machine Learning (ML) due to their recent contributions in the methodological developments for diverse problem domains, including computational biology [7, 8], cyber security [9,10,11,12], disease detection [13,14,15,16,17,18,19] and management [20,21,22,23,24,25], elderly care [26, 27], epidemiological study [28], fighting pandemic [29,30,31,32,33,34,35], healthcare [36,37,38,39,40], healthcare service delivery [41,42,43], natural language processing [44,45,46,47,48], social inclusion [49,50,51] and many more. This paper is a detailed study of different automated CHA techniques and technologies available in the literature, their challenges, and the future prospects in objectively assessing cognitive health using state-of-the-art technology. The notations used in the paper is listed in Table 1.
Existing Studies
Misdiagnosed or undetected CI can lead to a rapid decline in the mental health of a patient and can rapidly progress to AD. To automate the assessment process, many researchers have been working to develop systems that can use different technologies to automate CI assessment leading to accurate and timely prediction of MCI so that it’s progression can be slowed down and effective management of the disease can be put in place to improve the quality of life of the patients. Several reviews have been found on similar topics assessing the validity of different AI tools and techniques used for cognitive health management.
Authors in [52] reviewed different AI techniques used in detecting AD from MRI images. They discussed in detail the different MRI image-based datasets commonly used by researchers and the most prominent AI tools, like ML and CNN, used for feature extraction and classification of AD. The authors have explored only the image-based datasets and their efficacy in CHA.
Authors in [53] provided another review that discusses the AI approaches to predict cognitive decline in the elderly. The authors highlighted six different areas of features and datasets that researchers have used in employing AI techniques. These include socio-demographic data, clinical and psychometric assessments, neuroimaging and neurophysiological data, Electronic Health Record (EHR) data and claims data, various assessment data (e.g., sensor, handwriting, and speech analyses), and genomic data. Furthermore, the authors discussed about the future prospects and the ethical use of AI in CHA. One limitation of the paper was that it lacked an in-depth analysis of different AI tools and techniques.
Authors in [54] reviewed the different AI techniques like ML-supervised learning, unsupervised learning, Deep Learning (DL), and Natural Language Processing (NLP) to predict AD or develop diagnostic markers for AD. The authors discuss how researchers have used AI techniques to obtain and analyse data to predict AD. Augmented Reality (AR), Kinematic Analysis (KA), and Wearable Sensors (WS) have also been discussed as tools to establish digital biomarkers for AD. In this review, the discussion on AI techniques is brief and does not include the state-of-the-art AI tools for disease prediction and management.
Authors in [55] reviewed computer-aided diagnosis tools used in assessing and evaluating areas such as: 1) in the use of AI tools for early detection of AD, 2) in identifying MCI subjects who are at risk of AD, 3) in predicting the course and progression of the disease and 4) the use of AI in precision medicine for AD. It discussed the promising work of different researchers using ML, DL, NLP, Virtual Reality (VR),WS, etc., to analyse patient data. The authors discussed the possibility of precision medicine for AD using AI by highlighting novel research based on layered clusters.
Authors in [56] presented a detailed review of existing automated approaches used in neurodegenerative disorders, like AD, Parkinson’s Disease (PD), Huntington’s Disease (HD), Amyotrophic Lateral Sclerosis (ALS), and Multiple System Atrophy (MSA). It also includes a discussion on open challenges and future perspectives. The authors analysed symptom-wise computational methods that different researchers have used along with the classification methodologies and datasets used for each disease and their symptoms.
Authors in [57] presented a systematic literature review of 51 articles on using AI, speech, and language processing to predict CI and AD. The author categorised the features into text-based and speech-based and further elaborated on the speech and text-based datasets that different researchers have used in identifying digital biomarkers that can be assessed using ML. A detailed analysis of the current research regarding their objectives, population samples, datasets and datatypes used, feature extraction methods, pre-processing techniques, etc. have been performed. Unfortunately, the authors just explored one modality and analysed the role of natural language and speech processing in the prediction and diagnosis.
Authors in [58] reviewed 11 research studies that discuss different computerised assessment techniques like tablet-based cognitive assessment, remotely administered tablet and smartphone-based cognitive assessment, and other recent data collection systems and analysis procedures like speech, eye movement, and spatial navigation assessment. A study of their validation with AD biomarkers has been presented along with their potential in evaluating AD. This work just discussed the data acquisition techniques. It lacked a discussion on the potential barriers to implementation, future challenges, and data security issues.
Authors in [59] provided a systematic literature review on the significance of technologies producing digital biomarkers used for home-based monitoring of MCI and AD. The authors gathered research and categorised them according to the mode of automatic data collection via different sensors and other modalities, e.g., data from embedded or passive sensors in homes and cars, data from dedicated wearable sensors, data from smartphone-based automated interviews, Nintendo Wii, and VR. Also data obtained from secondary sources of everyday computer use and Activities of Daily Living assessment were included. Very little discussion regarding the different AI tools used in the domain and the future challenges was presented in this paper.
Authors in [60] also reviewed 16 papers discussing 11 assessment tools conducted over a computer, laptop, tablet, or touchscreen. The authors then evaluated all the outcomes and compared the accuracy, sensitivity, specificity, and Area Under the Curve (AUC) measure. The Authors in [61] compared different ML algorithms to detect and diagnose Dementia. The authors suggested a model whereby ML and MRI scan images can yield the best results. Authors in [62] reviewed different applications used for medical assessment of dementia and AD and presented a summary of features evaluated by different AD screening apps. It further listed the cognitive assessment tools used in various apps and their validity measures. It then reported their sensitivity and specificity measures to evaluate the performance of these screening tools. The limitation of this paper is that the authors have only explored the use of smartphones for cognitive assessment.
Table 2 concisely compares the important survey papers and highlights the existing surveys’ gaps, focusing on the different AI techniques used to diagnose and predict CI using different data modalities. Considering the limitations and gaps of the existing surveys, there is a high need for research focusing on different the state-of-the-art AI techniques for a precise and timely diagnosis of the disease to slow down cognitive decline and it’s progression.
Research Methodology
In formulating this research paper, we employed the systematic literature review process defined by the benchmark PRISMA approach. First, the existing surveys in the domain of AI-based CHA assessment were analysed to identify the research gaps. Keeping the research gaps in mind, an electronic search was performed using the keywords “Cognitive” and/or “Assessment” and/or “Impairment” and/or “Mild Cognitive Impairment” and/or “Dementia” and/or “Alzheimer’s” and/or “Artificial Intelligence”, over the different digital repositories like Google Scholar, IEEE, Elsevier, Springer and ACM to find papers relevant to our study. Only high-quality peer-reviewed articles from reputed journals, conferences, workshops, and books were selected. Papers were included based on relevancy, accuracy, and timeliness. All research studies before the year 2018 were excluded from the research. After performing the initial search, screening and shortlisting were done systematically. Initially, articles were screened based on their titles and relevance to the research objectives, and then they were further shortlisted by carefully reviewing the abstracts and contributions of the articles. Low-quality and irrelevant papers were excluded at each stage. The selected articles were then scrutinised. Finally, the included articles referenced in the survey have been selected after scrupulously and meticulously studying them.
Motivation
With the prevalence of cognitive diseases, the need for timely diagnosis and management is highly essential. At the current pace, it is estimated that by 2050 the cost of disease management and care services is expected to increase 9.5 times worldwide, which amounts to approximately 9.1 trillion dollars [63]. Delayed or missed diagnosis has been the leading cause of the prevalence of this disease. It has been observed that MCI can result in an early onset of AD and dementia. Early, timely and accurate diagnosis is the key to managing and delaying the onset of the disease and reducing the related health risks. We performed extensive research to compile different studies by authors in the domain of early detection of CI. Existing surveys lack an in-depth search of all the AI techniques used for MCI, AD, and dementia detection using different data acquisition means and modalities. Existing systematic literature reviews and surveys focus on either a single data modality like neuroimaging, speech, language datasets and explore the different AI techniques used in detecting and diagnosing MCI, AD, and dementia. Some research papers briefly overview the different AI techniques employed in the domain. Only two researchers explored the different datasets that researchers have used.
To the best of our knowledge, no comprehensive survey has been compiled that discusses all the different AI techniques applied to different data modalities used for automated CHA, their challenges, the state-of-the-art technology, open issues, and future directions. Our work will assist future authors in developing solutions covering the identified research gaps and enhancing the CI assessment process so that the progression to AD can be reduced by timely management and intervention. Furthermore, this detailed survey will help identify the evaluation techniques that are most effective and accurate in the early detection of CI and predicting cognitive disease progression.
Contributions
This paper is a detailed survey and analysis of all major AI-based CHA techniques and approaches. It also highlights the challenges and future directions in the domain. The automation of cognitive assessment is rapidly progressing, transforming the process into an accurate, dependable, and objective one. Documenting and summarising all the findings into one comprehensive survey is the need of the hour so that future researchers can explore the research gaps and further improve the assessment process. Among the main contributions of the current paper are the following:
-
1.
We analyse the existing clinical cognitive assessment tools, their drawbacks, and the evolution of cognitive assessment tools and techniques through the incorporation of AI techniques.
-
2.
We highlight the different popular data acquisition methods currently used in obtaining data regarding CI individuals.
-
3.
We identify and evaluate the latest advancements in technology which can uplift the health industry and reduce the financial burden caused by misdiagnosis of CI and AD.
-
4.
We explore the different AI techniques researchers use like conventional ML, DL, and NLP.
-
5.
Lastly, we discuss the challenges and future directions in the domain of CHA, hence presenting an overview of the research gaps in the area.
Organisation
“Introduction” section introduces the paper which includes a discussion on the present background of CHA, a look at the existing literature reviews and surveys on similar topics, our motivation to compile this survey, and its contributions to the research domain. “Background and Enabling Technologies for Automated Cognitive Health Assessment” section explains all the enabling technologies used for CHA, like IoT and cloud computing. This is followed by “Artificial Intelligence Techniques for Assessing Cognitive Health” section, which provides a detailed discussion of the different AI techniques researchers use for CHA. “Data Acquisition Channels” section explores different data acquisition methods used in acquiring data to analyse using AI techniques. “Cognitive Health Assessment Datasets” section presents a comprehensive table highlighting the different datasets researchers have used for CHA. Finally, “Challenges and Open Issues and Future Directions” section discusses the challenges, open issues, and future directions in the field of AI for CHA. Figure 2 shows the structure of the paper.
Background and Enabling Technologies for Automated Cognitive Health Assessment
This section presents the existing background and enabling technologies for automated cognitive health assessment.
Present Background of Cognitive Healthcare Assessment
The current diagnosis of cognitively impaired individuals is a step-by-step process. A patient undergoes an initial assessment at a local clinic. Further testing is done at the neurological centre. Various screening tools like self-administered questionnaires, interview-administered assessments, telephone-based assessments, and iPad assessment versions have been introduced to screen patients with cognitive symptoms [64]. According to PubMed, roughly eight cognitive domains are evaluated in a cognitive assessment: sensation, perception, motor skills and construction, attention and concentration, memory, executive functioning, processing speed, and language and verbal skills [65]. To assess these domains, several screening tools have been used in the past ten years [64, 66]. Table 3 lists some of these. Furthermore, Fig. 3 presents the cognitive assessment domains.
The available objective methods for assessing Cognitive Impairment are frequently based on interview-based and self-administered questionnaires or are conducted in the clinical setting where an artificial environment can influence response, thus increasing the possibility for biases and misinformation. Automating the assessment and prediction process is the key to timely diagnosis and management.
Enabling technologies (i.e. Internet of Things (IoT), Cloud Computing, Edge Computing, Internet of Medical Things (IoMT), Big data Analytics, 5G and Beyond, Ubiquitous Computing, and Virtual Reality) play a vital role to improves the way of living style of cognitively impaired individuals. This section provides the enabling technologies for automated cognitive health assessment.
Internet of Things
IoT is one key enabling technology for automated cognitive health assessment. IoT is a vast linked network of various components that enable smart devices to detect, distribute and evaluate data and deploy these devices in different domains like food chain management, healthcare monitoring, smart homes, smart cities, and environmental mentoring [69, 70]. A considerable increase has been in IoT devices like smartwatches, sensors, smoke detectors, heartbeat monitors, smart homes, and smartphones. There were 8.4 billion IoT devices in 2020, which will touch 20.4 billion in 2022 [71]. IoT is significantly growing in the healthcare sector. The IoT industry is slowly picking up pace due to many issues, such as scalability, accepted standards, heterogeneity, and integration in IT infrastructure. To monitor patients’ health, wearable devices are gaining popularity in IoT. For the duration of medical examination and treatment, wearable devices are connected to an individual’s body to collect patient health data.
Smart Home
IoT significantly improves the living style by introducing smart homes. A smart home provides the ability to perform daily routines from simple to complex tasks to cognitively impaired individuals. Various sensors embedded in smart homes, like temperature, motion, heat, and light, are intelligent enough to make decisions for the smart home environment. In the healthcare system, smart homes are used for activity recognition to promote sustainability and improve the lifestyle of cognitively impaired individuals [72]. The activity recognition process recognises the individual’s daily activities, such as preparing food, eating, drinking, toileting, reading newspapers or books, walking, sleeping, watching TV, and home cleaning. Several techniques are available for activity recognition, like on-body intrusive sensors, on-body non-intrusive sensors, and wearable devices [73]. Still, smart homes and smartphones are frequently used due to their non-obtrusive behaviour.
The motivation for using IoT for a cognitive health assessment is to provide immediate care and treatment to cognitively impaired individuals. IoT devices help keep track of cognitively impaired individuals’ daily activities to perform daily life tasks efficiently. IoT devices also allow healthcare professionals to monitor cognitively impaired individuals remotely.
The healthcare sector faces several significant challenges in the security and privacy of individual data. IoT devices help collect data for efficient treatment, but private data may be lost. The availability of IoT devices in the health care system makes personal data a more valuable target for cyber attackers. Data Analysis is also a challenge for IoT devices because the growing number of sensors, smart devices, and connected things indicate that a vast volume of data is being generated on daily bases; thus, it is challenging for IoT in the healthcare system to be able to analyse data and extract exact data for treatment. The connectivity of smart devices has also become an issue nowadays. A disruption in internet connectivity interrupts the interaction between healthcare professionals and cognitively impaired individuals. This can be disastrous for the monitoring process. IoT is a vast linked network that is used for cognitive health assessment. The primary purpose of using IoT is to assess people’s mental health and provide immediate care and treatment. In the healthcare system, IoT has gained immense importance. The healthcare system faces several challenges related to privacy and security, data analysis, and internet connection interruption.
Cloud Computing
Cloud computing has become the basic need of the healthcare industry due to complete access, automation backups, and disaster recovery options. Cloud computing provides an adaptable architecture where data is accessible from different locations without losing it [74, 75]. In the health care system, cloud computing offers a new level of safety and low-cost treatment. It helps healthcare professionals conduct voice and video appointments and access patients’ required private data for better treatment.
Cloud computing is impressively helpful in the healthcare sector. Telemedicine is the major motivation for using cloud computing for cognitive health assessment, as healthcare professionals can access a patient’s clinical history and mobile data through mobile telemedicine applications [76]. Thus cloud computing provides an opportunity to improve fast services, easily share information, improve operational efficiency and streamline costs.
Cloud computing is an emerging technology and faces many challenges in information handling in the healthcare industry. Security is the most common concern of cloud computing in healthcare. HIPPA compliance, data protection, and infrastructure changes are a few problems in cloud computing.
Cloud computing is a new model for computing resources and self-service internet infrastructure. Cloud computing improves patients’ immediate care and treatment at a low cost, and healthcare professionals can easily access patients’ clinical histories. Cloud computing also faces security and privacy problems in the healthcare sector. It is noticed s that cyber-attacks have been patiently reduced by compressing cloud computing to other storage magnesium [77].
Edge Computing
Edge computing and IoT are potent ways to analyse data in real-time quickly. Edge computing is a distributed computing framework in which data is collected, stored, processed, and analysed near the data source instead of in a centralise data-processing warehouse. Edge computing allows IoT devices to collect and process data at the edge where it is collected. Cloud computing becomes an inefficient structure for vast data processing and analysis collected from IoT devices [78]. Thus, edge computing becomes a basic need for moving data and services from centralised nodes to the network’s edge.
In the healthcare sector, edge computing plays a vital role. The primary motivation for edge computing is data efficiency, increased data security and ethical integrity, and reduced dependency on remote centralised servers [77].
Edge computing is also facing some major problems in the healthcare industry. It has been noticed that the primary challenges are coping with large data sets produced by medical sensors, patients’ personal medical data legal issues, and the integration of artificial intelligence in a 5G environment. Some challenges in intelligent manufacturing include operation and maintenance, scalability, and reliability of the data centres [79].
The exploration of edge computing is moving rapidly. Edge computing has become a basic need for moving data and services to the network’s edge due to limitations in the cloud computing platform. Edge computing enables real-time medical equipment management and helps monitor patient history like glucose monitors and blood pressure cuffs remotely, alert medical professionals regarding patient health [80, 81]. Security and lack of consistent regulation is the noticeable challenge for edge computing.
Internet of Medical Things (IoMT)
The internet of medical Things (IoMT) is a huge network of physical devices integrated with sensors, network connections, and electronics, enabling the devices to collect patient data for medical purposes. The IoMT has been called “Smart Healthcare” as the technology for creating a digitised healthcare system, connecting available medical resources and healthcare services. IoMT technology enables virtually any medical device to connect analyses and send data across digital devices and non-digital devices such as hospital beds and pills [82]. IoMT improves healthcare quality while reducing costs. IoM is an infrastructure of network software, health system devices, and service-connected to collect user data.
The major motivations for using the IoMT are Real-time data care systems and healthcare analytics. The IoMT in the medical field allows doctors to manage operations data systematically. Further to healthcare experts, the data is then examined and delivered to offer a better health solution for patients in real-time [83]. The real-time data care system can give the best health solution.
IoM is also facing challenges in the healthcare field, like other enabling technologies. Security and Privacy of IoT data are still on top. IoM has introduced other significant challenges like lack of standards and communication protocols, errors in patient data handling, data integration, managing device diversity, and interoperability [84]. IoT devices have provided many ways to improve data collection and quality. IoM is considered the best option for collecting real-time patient data and monitoring. IoM has connected doctors, patients, researchers, manufacturers, insurers, and industries [85]. In the future, it is estimated that bringing the IoT into medicine will help in more substantial, healthier, and easier patient care.
Big Data Analytics
Big data is vital in improving healthcare delivery, helping to measure and evaluate comprehensive healthcare data [86]. Much medical data must be integrated and accessed intelligently to support better and fast health care. Big data introduced a new network by measuring and monitoring data processes digitally, and we can compare data more efficiently. Such insights facilitate streamlined workflows, greater efficiencies, and improved patient care. Big data only benefits healthcare when structured, relevant, intelligent, and accessible.
The primary motivation to use big data analytics in healthcare is to provide insight from large data sets and improve outcomes at a low cost. Big data analytics reduce medical errors, detect disease early, and provide more accurate treatment with real-time data collection. Big data analytics is associated with many challenges like data collection, cleaning, security of collected data, storage, and real-time updates [87]. Accessing big data from external sources is also a big challenge. The Healthcare sector is still concerned with privacy and security.
It has been noticed that big data analysis plays a major role in healthcare. In the healthcare industry, a massive amount of data is generated daily, stored in different locations, and expected to be efficiently accessed by healthcare professionals; thus, big data can potentially improve the quality of healthcare delivery at low costs [88]. Meanwhile, big data analysis faces security and privacy issues, but at the same time, it is considered the best technology to handle vast data effectively.
5G and Beyond
The promise of digital health is materialised without switching to fifth-generation (5G) cellular technology. 5G network directly contribute to better diagnosis and faster triage with higher bandwidth and low latency, saving lives [89]. 5G connected wearables (e.g. smartwatch, bands SGPS/GPRS body control, VR headset, and smart glasses) will facilitate real-time data streaming [90]. 5G will enrich everything from prevention to treatment to rehabilitation, teaching, and mentoring.
The primary motivation for using 5G networks is speed. 5G networks will allow devices to download more than 1 GB of data in one second; it could be 100 times faster than today’s 4G. 5G promises high bandwidth and low latency. 5G can make many areas of telehealth, remote surgery, and continual treatment information efficient.
Health care is about communicating and connecting better. 5G will cause a paradigm shift in healthcare. However, with the implementation of the 5G network, many companies and individuals are concerned about the loss of sensitive information and data. Data mismanagement, medical identity theft, health privacy, and data security are the big challenges for 5G [91].
5G network can make communication easier, faster, and more real-time. In the healthcare sector, the 5G network can boost collaboration between healthcare professionals and make them collaborate during surgery and scans to provide faster care and health treatment to patients [92]. 5G can make smart hospitals efficient and reliable at low latency. 5G directly supports telemedicine in the aspect of security and privacy.
Mobile Computing
People can access data and information from everywhere through mobile computing. Mobile computing forms like Cell phones, tablets, wireless laptops, and push-to-talk devices make life easy. Mobile computing provides more security than other enabling technologies. Mobile computing strengthens the IoT health-based system with various services, applications, third-party APIs, and mobile sensors [93].
Mobile Computing is used to support healthier lifestyles. Mobile computing allows patients to stay out of hospital beds and give them home-based healthcare services at a low cost. Mobile computing monitors patient health in surgery, spectroscopy, and Magnetic Resonance Imaging (MRI) [94]. It is helpful to maintain and secure data storage and diagnostic databases.
Mobile computing is also facing many challenges, like other enabling techniques. The major challenges are the usability of mobile applications, system integration, data security and privacy, network access, and, most importantly, reliability.
Mobile computing links the human brain and computers to improve the healthcare system. The computer system can tackle complex decision-making processes by learning patterns and behaviours and becoming more intelligent [95]. Mobile applications are vital in giving the cognitively impaired a healthier lifestyle.
Virtual Reality
Virtual reality (VR) is a computer-generated simulation that allows users to simulate a situation and experience of interest using a VR headset [96, 97]. VR can be similar to or completely different from the real world. VR is revolutionising the healthcare industry by using different VR applications. Moreover, VR improves the knowledge and skills of medical professionals and students. Currently, many medical organisations are paying attention to various VR medical training. VR plays a vital role in different areas of healthcare, facilitating medical trainers, treatment of phantom limb pain, VR therapy, treatment of patients, Relief tools for doctors, helping autistic children and adults, pain management, VR surgery simulation, and real-time conferences.
VR has many drawbacks in the healthcare sector, like the high cost of VR software and equipment, VR addiction, Disorienting users, lack of proper trials, and insufficient training.
Healthcare is the most important field, whether in the past, present, or future. VR is considered the best option for cognitive health assessment, establishing symptom correlates, identifying differential predictors, and identifying environmental predictors [98].
Artificial Intelligence Techniques for Assessing Cognitive Health
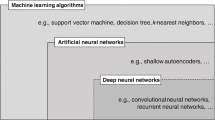
Artificial Intelligence (AI) is a scientific field that falls under the computer sciences discipline. AI’s primary focus is on building systems or machines. These machines help carry out tasks typically involving decision-making and human intelligence. AI algorithms learn from datasets and continuously update their learning on the availability of new data [99]. The output of AI models depends on various factors, including the size of the dataset, feature selection, and parameter selection. AI techniques are designed to handle large volumes and the complexity of datasets. These AI techniques produce ideal results in various complex examples and are more accurate and efficient than human beings [100]. The development of various AI techniques has contributed effectively to early detections, disease diagnoses, and referral management because experts are limited regarding performance, knowledge diversity and daily exertion can also affect their performance. AI-based systems are addressing these limitations. Machine learning (ML) is a sub-category of AI that learns from the data without being explicitly programmed. ML algorithms are being used in solving problems with high level of similarities within data [101]. The advancement in machine learning techniques has also changed the field of computer-assisted medical image analysis and computer-assisted diagnosis (CAD). ML techniques have shown promising results in the timely prediction of disease. In this section, various ML techniques, including deep learning [102], supervised and unsupervised approaches [103], Reinforcement Learning [104], Natural Language Processing [105], and Computer Vision [106] have been discussed to assess the cognitive health of a person.
Supervised Learning Approaches
Supervised Learning (SL) approaches need labelled data (e.g. diagnosis of cognitive disorder vs healthy people). This labelled data is given as input to a model, which learns from this labelled data. Based on the learning, the trained model predicts the output [103]. The correctness of the SL models depends on the “ground truth” behind the labelled outcomes. Figure 4 illustrates how supervised learning occurs. Supervised learning techniques are divided into supervised deep learning techniques and ML techniques. Below we have given a comprehensive tour of state-of-the-art supervised learning approaches.
Machine Learning
Supervised machine learning algorithms are designed for regression and classification-based problems. Various supervised learning techniques have been used for assesment [72, 107,108,109,110] and detection of cognitive health diseases [111,112,113,114, 114,115,116,117,118,119,120,121,122,123,124]. Popular examples of supervised ML techniques include Bayesian, Decision trees, Support Vector Machine (SVM) and its variants, Logistic regression, and many more. Authors in [113] a supervised machine learning method are discussed to detect patients with cognitive disorders. It utilised a multifold Bayesian kernelisation approach and classified the patients into three classes, namely Alzheimer’s, MCI, and normal controls. This method produced good results in identifying Alzheimer’s and normal controls but not in identifying patients with MCI. Catá Villá [125] Bayesian classifier used for Alzheimer’s disease detection. This technique achieved accuracy up to 92.0% when applied to the MRI dataset [126]. Another technique that used a Bayesian classifier to identify Alzheimer’s disease is presented in [112]. However, it used PCA and Linear Discriminate Analysis to extract features before applying Bayesian. Extracting features with PCA and LDA boosted the performance of the proposed model. Input images used in this method are PET and Single Photon Emission Computed Tomography (SPECT).
Authors in [115] utilised supervised learning and used Random Forest to classify multi-class Alzheimer’s Disease with an accuracy of 92.4%. Laske et al. [114] proposed a Support Vector Machine to classify subjects with Alzheimer’s disease. This technique scored an accuracy of 81.7%. Authors in [116] PET and SPECT images are used for the detection of cognitive health of a patient using SVM. It has been shown that classification using SPECT images achieved higher accuracy than PET images. SVM is used for both regression and classification-based problems. SVM and its variants show promising results in identifying people with cognitive health issues. SVM-based Alzheimer’s detection [117] showed that instead of using a single input image, two types of images (MRI and FDG-PET) are combined for input to SVM could significantly increase the detection accuracy of Alzheimer’s patients. Input images used in [117] are taken from ADNI and Leipzig Cohorts datasets. SVM-based Alzheimer’s detection model [127] focused on Hippocampi input and distinguished patients with Alzheimer’s disease from healthy controls with an accuracy of 94.6%. Zhang et al. [128] classified elderly subjects into three classes, Alzheimer’s, MCI, and Normal control. They utilised 5-fold cross-validation for KSVM-DT. Authors in [129] combined a non-negative matrix factorisation with an SVM classifier to distinguish between patients with Alzheimer’s and healthy controls. Authors in [130] used combined MRI and SPECT images with SVM, and good results were obtained for disease identification. Histogram and Support Vector Machine are combined to distinguish patients with Alzheimer’s Disease from other normal subjects. Using MRI images as input, accuracy, sensitivity, and specificity of 94.6%, 91.5%, and 96.6%, respectively, proved the technique’s novelty. Another hybrid technique is proposed in [118], which stacked Wavelet Transform and SVM together for diseased and normal patients classification. It produced accuracy, sensitivity, and specificity of 80.44%, 87.80%, and 73.08%, respectively.
Logistic Regression performs classification for Alzheimer’s disease by looking into the probabilistic value returned through the logistic sigmoid function. Authors in [119] have shown that timely detection and progression of Alzheimer’s disease can be detected with the help of logistic regression classifier. A two-staged methodology is presented in [120], which used Logistic regression to detect dementia. Authors in [131] introduced trace ratio linear discriminate analysis (TR-LDA) to distinguish patients with Dementia disease from healthy subjects. Fisher Linear Discriminant (LDA) develops a linear discriminant function which results in a minimum error. This hybrid technique worked well and achieved an accuracy of 90.01%.
Meng et al. [132] found new areas of the brain which are helpful in the early diagnosis of Alzheimer’s. Feature extraction described by them is a fusion of voxel-based features extracted from MRI and eigenvalues of Single Nucleotide Polymorphisms that can identify the important brain voxels that can classify AD, early mild cognitive impairment, and healthy control by using single kernel SVM and standard multi-kernel SVM. Another algorithm for early detection of Alzheimer’s is introduced in [121]. This technique is built upon an operational research’s subdiscipline Multicriteria Decision Aid (MCDA) classification method. To adjust the weights and thresholds of the MCDA classifier, a Genetic Algorithm engine and ELECTRE IV algorithm are deployed. Authors in [108] utilised the three segments of the MRI dataset: Corpus Callosum, Hippocampus, and Cortex. SVM is used to classify the controls and attained an accuracy of 91.67% in the early Alzheimer’s disease finding.
Authors in [111], used resting-state functional magnetic resonance imaging (rsfMRI) to compute the time series of some anatomical regions and then applied the Latent Low-Rank Representation method to extract suitable features. For the classification task, SVM was used to classify all the input patients in either of the classes, i.e. MCI and healthy controls. The obtained classification accuracy for this method is more than 97.5%. Experiments are performed using images of 43 healthy subjects, 36 mild cognitive impairment patients, and 32 Alzheimer’s patients.
In [109], Clinical Assessment using an Activity Behaviour (CAAB) based approach is described where a person’s health is assessed by detecting the change in their behaviour. CAAB considered the activity-labelled sensor dataset to find activity performance features. From these features, it then extracted statistical activity features (variance, autocorrelation, skewness, kurtosis, etc.), which are given as input to an SVM to calculate the cognitive and mobility scores. Authors in [72], used supervised learning to examine the simple daily living activities and complex daily living activities performed by smart home residents. This technique was assessed based on temporal features and classified the individuals into healthy, Mild Cognitive Impairment, and dementia. PCA was used to select the most valuable features. This feature selection step is beneficial in avoiding overfitting, and at the same time, it improves accuracy and training time. Selected features are then used to classify the individuals into various classes. Classification models discussed in [72] are Decision Tree, Naıve Bayes, Sequential Minimal Optimization, Multilayer Perceptron, and Ensemble AdaBoost. Among these classification models, Ensemble Adaboost best predicted individuals with cognitive impairment. Authors in [110] showed that a person’s cognitive health could easily be assessed based on the performance of different tasks. For this reason, the Day Out Task (DOT) is designed. DOT includes various activities and subtask performs to complete these activities. Various participants are asked to perform these tasks, and their response is captured using sensors. Features collected from these sensors are then fed into a machine learning algorithm to determine the quality of an individual’s performance based on DOT. Supervised and unsupervised machine learning techniques are used to classify the collected features. The supervised approach uses SVM with sequential machine optimisation and bootstrap aggregation. For the unsupervised approach, PCA is used to reduce the dimensions. Min-max normalisation is then applied to transform the variables to a uniform range. SVM is finally used to classify the participant into various classes depending on the sensor-based activity features. Classes defined in this work are dementia, mild cognitive impairment, and cognitively healthy.
The technique presented in [107] detected behaviour change through smartwatch data. These smartwatches are designed to collect data continuously. The main goal is to detect and quantify the difference in overall behaviour activity patterns promoted by the intervention. Permutation-based change detection (PCD) algorithm detects the change in activity pattern. The autoptimisationd a Permutation-Based Change Detection (PCD) method. A random forest algorithm is applied to detect behaviour normalisation. The algorithm calculated the change in behaviour between time points with an accuracy of 0.87.
Authors in [133] proposed an onset detection model for dementia and MCI. Motion, power usage, and other related sensors detect activity in a smart environment. The dataset used is self-created and collected from an IoT-enabled smart environment. Features are classified using several supervised machine learning algorithms and Ensemble RUSBoosted Model. These models have achieved 90.74% accuracy in detecting the onset of dementia. Limitations of this work include limited feature values, and while collecting IoT-based data, security and privacy concerns are not considered. Authors in [134] used a supervised dictionary learning technique that applies Correlation-based Label Consistent K-SVD (CLC-KSVD) on extracted patches spectral features from EEG.
Anomaly detection techniques help to find the unrelated sample, also called an anomaly, from the given data. Such anomaly detection techniques are applied to the features to predict a sudden change in a person’s behaviour, which indicates a cognitive health issue. In [135], an anomaly detection method is proposed, which performs well than traditional anomaly detection methods. This algorithm is designed to provide indirect supervision to unsupervised methods. An Indirectly Supervised Detector of Relevant Anomalies (Isudra) detects anomalies from time series data. Isudra employed Bayesian optimisation to select time scales, features, base detector algorithms, and algorithm hyperparameters.
Workplace stress is one of the reasons for producing cognitive health issues. Therefore, Alberdi et al. [136] collected data from an office setting and applied various machine learning techniques, Naïve Bayes, Linear SVM, Ada boost, and C4.5, to this data. It is found that mental stress and workload levels define an employee’s behaviour change. Also, computer-use patterns combined with body posture helped predict a worker’s cognitive health.
For dementia patients, repeating the same task, e.g. taking medicine and eating a meal, could be dangerous. Therefore, these repeated tasks must be recognised correctly. To recognise the similar behaviour of a person with dementia. Authors in [137] integrated hand movements with indoor position information to identify the activity performed. Data is collected with the help of wearable sensors. In the DPMM-Layer, the hand’s movements are clustered based on feature values, so each hand’s movement is associated with one specific cluster. The indoor position is detected on the Bluetooth sensor’s data using the supervised algorithm Random Forest and Finite State Machine to recognise the individual’s position. The precision and recall of “eat” is 92.1% and 97.5%, and for “having Medicine” are 99.1% and 99.5%, respectively.
Authors in [138] discussed the relationship between behaviour and cognitive health. A set of digital behaviour markers is developed to predict clinical scores from activity-labelled time series data. Data is collected by using data collected from ambient and wearable sensors of 21 participants in a smart home environment. These digital behaviour markers aim to predict clinical scores for the data collected. Mobile-AR and Home-AR algorithms are used to label activities in real time. Gradient boosting regressor is used to predict the clinical score.
Deep Learning
Deep Learning is a subset of ML that shows promising results when the input data is unstructured and complex. The deep learning algorithm requires input data in large volumes. Deep Learning models consist of multiple layers, each with a unique way of interpreting the data. Spasov et al. [139] discussed a deep learning algorithm that took multiple sorts of data as input. The input data comprised structural magnetic resonance imaging, neuropsychological, demographic, and APOe4 genetic data. This approach used a deep convolutional neural network framework involving grouped and single convolutions. The most noticeable characteristic of this deep learning model is that this model can simultaneously classify the individuals into Mild Cognitive Impairment vs Alzheimer’s Detection and Alzheimer’s Detection vs healthy subjects and attained classification accuracy of 86%.
Choi and Jin [140] developed a deep learning system to evaluate a patient’s cognitive health. They considered 3D-PET images as input. This system used CNN to identify a person’s cognitive decline. The overall accuracy of this algorithm is 84.2%. Habuza et al. [141] came up with a model to detect the level of cognitive decline using MRI images. This model is based on a Convolutional Neural Network (CNN) classification model to estimate the level of cognitive decline. The architecture of this CNN model is similar to AlexNet. The T1-weighted MRI images dataset is pre-processed before being input to the CNN. To draw a line between healthy people and Alzheimer’s disease people, the Predicted Cognitive Gap is used as the biomarker. The proposed model is tested on a dataset that includes 422 healthy control and 377 Alzheimer’s disease cases. The performance of the proposed solution is 0.987 (ADAS-cog), 0.978 (MMSE) for averaged brain and 0.985 (ADAS-cog), 0.987 (MMSE) for the middle sliced image.
Authors in [124] analysed the effectiveness of ADNI data in determining how much the classification models can differentiate people with various cognitive diseases by classifying them into three classes. Multi-Layer Perceptron (MLP) models and a Convolutional Bidirectional Long Short-Term Memory model are explored in [124]. MLP model outperforms the ConvBLSTM model with an accuracy of 86%. Authors in [142] utilised the attention mechanism in deep network architecture to detect early-stage Alzheimer’s. The model is tested on a self-created dataset. Authors in [143] proposed the LeNet-5 deep model to distinguish Alzheimer’s patients using functional MRI (fMRI). The model produced a classification accuracy of 96.85%.
Authors in [144] predicted the existence of Alzheimer’s disease using multiple types of input data. The unique feature of this paper is that it presented deep learning models that use image and non-image data to detect Alzheimer’s disease. The input data consisted of socio-demographic, clinical notes, and MRI data. Deep convolutional Neural Network ResNet-50 predicts the clinical dementia rating (CDR) presence and severity from the input MRI whereas gradient boosted machine predicted Alzheimer’s using non-image data. The key limitations of this study include only 416 individuals whose MRI images are included in the dataset. Each image is composed of 51 selected slices of each patient’s MRI. The selection of slices is made carefully because eliminating a few slices may eliminate slices that help diagnose Alzheimer’s disease. In [145], another deep CNNs is proposed, which runs on MRI scans of the ADNI dataset. This deep model gave output by classifying each MRI image in either of the four classes, namely Alzheimer’s, Mild Cognitive Impairment, Late mild cognitive impairment, and healthy person. Various deep CNNs are tested, like GoogLeNet, ResNet-18, and ResNet-152; among these CNNs, GoogLeNet produced the highest accuracy of 99.88%. Authors in [122], a 3-dimensional VGG convolutional neural network is tested on two publically available datasets, namely, ADNI and OASIS. This 3D model has the advantage of preserving information as data loss may occur during converting 3-dimensional MRI into two-dimensional images and processing them by two-dimensional convolutional filters. Classification accuracy achieved by this 3D model is 73.4% on ADNI and 69.9% on the OASIS dataset. These results show that the 3D model outperforms the 2D network models. Table 4 presents the summary of supervised studies for cognitive health assessment.
Critical Analysis: Supervised learning techniques used for cognitive health assessment cannot detect cognitive disease but are also very helpful in detecting the progression of such cognitive disorders. As we have seen in the studies mentioned above, both traditional ML and DL techniques produced exceptional results in cognitive disease detection; nonetheless, the main challenge of these techniques is the availability of labelled data with interventions from human beings. In a hospital setting, such labelled data is unavailable and cannot be easily labelled at run time. Labelling data requires much effort by experts. DL techniques take the lead in performance as compared to ML techniques. Also, if a deep model is trained on one dataset, transfer learning techniques are available to apply this trained model to another dataset without retraining it from scratch. However, it is notable that the quality of the output of the deep learning model mainly depends on the size of the dataset. Data augmentation has addressed this issue to some extent, but as the size increases, it also extends the training time. Therefore, the DL technique is a tradeoff between performance and training complexity. Overfitting is another challenge with supervised learning techniques. Therefore, if it is possible to collect a large dataset, then deep learning approaches will be a good choice; otherwise, ML approaches should be considered.
Unsupervised Learning Approaches
Unsupervised Learning (UL) algorithms work with unlabeled data. A dataset for the classification task consists of data from all the classes, and an unsupervised algorithm is not privy to this knowledge [146]. Instead, the algorithm searches unstructured data for features and classifies them together in clusters. Figure 5 shows how data can be classified using the unsupervised learning approach. In this way, the data is segmented into different groups. All the grouped items share some common characteristics. Some items are classified as irrelevant, which means they do not possess the properties of a specific group. If this data is comprised of medical-related data, then involving a medical expert is a must to derive the meaning of identified clusters. In the following section, state-of-the-art Unsupervised Learning Approaches are discussed for the assessment [147,148,149,150] and detection [151,152,153,154,155,156,157,158,159,160,161,162] of the cognitive health of a person.
Machine Learning
Designing an activity recogniser for in-home settings and continuous data is very challenging. This difficulty is even more enhanced when the home residents are more than one. Estimating the number of residents and identifying each resident’s activity in a smart home is an open challenge that needs to be addressed. To address this challenge, [149] introduced an unsupervised learning approach based on a multi-target Gaussian mixture probability hypothesis density filter. If [149] is applied in the smart home environment. It can improve the accuracy of cognitive health assessment algorithms.
Authors in [152] discussed an unsupervised Alzheimer’s detection approach using a finite mixture model. Input data comprises a cerebrospinal fluid, magnetic resonance imaging, and cognitive measures. Researchers in [153] discussed an anomaly detection model which recognised activity by first applying a probabilistic neural network on the pre-segmented activity data obtained from the sensors deployed at different locations in a smart home. H2O autoencoder is used to identify the anomalous from the regular instances of activities. Anomalies are further categorised based on the criteria such as missing or extra subevents and unusual duration of action. Datasets for training and test purpose are Aruba and Milan datasets. The accuracy of detected achieved is more than 95% in all activities. For Alzheimer’s detection, [151] suggested a technique that used KNN to segment the areas in the MRI image. Authors of [163] presented an unsupervised technique on MRI data to detect various phases of Alzheimer’s disease. Authors in [164] provided techniques for making various clustering procedures more efficient. The authors aimed to describe cerebrum muscle physiognomies for assessing Alzheimer’s disease in various phases. Rodrigues et al. [148] discriminate AD cases from normal subjects by utilising K-means clustering. They observed K-means as the best method for an unsupervised diagnosis of EEG temporal arrangements. Authors in [154] K-means is applied to different data features to divide the subjects into pathologic groups. “Bio profile” [155] is analysed to reveal the pattern for Alzheimer’s disease. Here the researchers used K-means clustering to detect AD on the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. Detection of biomarkers is important, and therefore [147] suggested an unsupervised learning mixture modeling method to detect biomarker patterns of Alzheimer’s patients from the Alzheimer’s Disease Neuroimaging Initiative database. Authors in [156] identified the groups of patients with signs of Alzheimer’s and divided the patients into three clusters where three showed low, medium, and high extrapyramidal burden. The importance of brain imaging is highlighted in [165], where the researchers diagnosed AD or vascular dementia using the unsupervised model on brain imaging.
Authors in [159] LSTM process electronic health records, a deep learning technique to differentiate between a healthy person and a person with mild Cognitive Impairment. The clustering of patients is done by using a denoising autoencoder to represent the patient data better. The output of this autoencoder is visualised and clustered using t-stochastic neighbour embedding. The accuracy of LSTM is 73%, and the F1-score is 0.43. [159] highlights that if temporal characteristics of the patient’s data are incorporated into the deep learning model, it gives greater results in predicting Mild Cognitive Impairment. Authors in [157] is another unsupervised technique that used K-means clustering to divide patients with Alzheimer’s into clusters. A cortical thickness-based clustering method is proposed in [166]. Here the clustering is performed on the data collected from 77 patients’ MRI, PET, and cerebrospinal fluid (CSF).
Gamberger et al. [167] applied a multi-clustering method to an AD dataset of male and female patients comprising 243 biological and clinical features. Authors of [168] observed three clusters in a 3D-MRI, dominated by medial-temporal atrophy, parietal atrophy, and diffuse atrophy. Authors in [158] propose a clustering-based technique to detect Alzheimer’s using pathological data. Patients often face sleep problem issues with cognitive impairment. To detect Alzheimer’s through sleep disorder, [169] took samples of 205 patients with AD from the Alzheimer’s Disease Patient Registry to investigate patterns of sleep problems. The authors applied hierarchical cluster analysis.
Deep Learning
An unsupervised Convolutional Neural Network (CNN) is discussed in [170], which is used to diagnose cognitive diseases. PCANet learned the features from one slice and three orthogonal panels of MRI images and then used K-means clustering, an unsupervised classification technique. Experiments are done on the ADNI dataset. Results generated through this model are auspicious, with an accuracy of 95.52% for AD vs MCI classification and 90.63% accuracy for MCI vs NC when only one slice of MRI is taken as input. When three orthogonal panels of MRI images are considered, the accuracy improved even more, 97.01% for AD vs MCI and 92.6% for MCI vs NC. Here NC represents standard control.
Authors in [160] used a Stacked autoencoder to extract latent features on a huge set of features obtained from MRI and PET images. This is an unsupervised model for the assessment of the cognitive health of a person. Authors in [171] used a Stacked autoencoder accompanied by supervised fine-tuning to classify Alzheimer’s, Mild Cognitive Impairment, and healthy controls. Experiments were conducted on the ADNI dataset. A deep 3D convolutional neural network prediction model of Alzheimer’s disease is presented in [172], which can learn generic features capturing Alzheimer’s disease biomarkers. The advantage of this technique is that it can be used on different domain datasets. An autoencoder is an artificial neural network used to learn efficient coding of unlabeled data and its variant stacked autoencoders and a softmax output layer to detect Alzheimer’s disease from MRI and PET images [173]. The technique presented in [174] is a novel method for a high-level latent feature representation from neuroimaging data via an ensemble classifier. Authors in [175] used fMRI data to distinguish between MCI and healthy controls. The use of deep AutoEncoder with HMM produced good results in prediction.
Han et al. [176] proposed a two-step unsupervised anomaly detection approach based on multiple-slice reconstruction. This technique showed promising results by detecting AD at a late stage with AUC 0.894. Alzheimer’s detection across different disease stages using MRI is presented in [177]. This algorithm can detect Alzheimer’s disease at various locations. Unsupervised Alzheimer’s detection using the deep convolutional generative adversarial network is discussed in [178]. It performed anomaly detection on brain MRIs to diagnose AD and achieved an accuracy of 74.44%. Authors in [162] explore the anomaly analysis of Alzheimer’s disease using PET images for GANs. A feature vector is calculated using CNN and DCN. ADNI dataset is used to perform experiments. Authors in [179] proposed an algorithm to generate images of the brain for different stages of Alzheimer’s disease. They used PET images, and the algorithm used was deep GANs. Authors in [180] used a restricted Boltzmann machine to classify cognitive normal and early mild cognitive impairment of Alzheimer’s disease by applying the Restricted Boltzmann Machine to fMRI data. Authors in [150] employed a Conditional Restricted Boltzmann Machine to simulate Alzheimer’s disease progression from a 1909 patient. Table 5 presents the summary of unsupervised studies for cognitive health assessment.
Critical Analysis: In the supervised learning section, we have seen various models’ superb performance in cognitive health assessment. However, we can not always give lots of samples with supervision. Most of the time, the machine needs to learn from the training data, and here comes the role of unsupervised learning. These unsupervised methods save time and energy in labelling the data. It is a powerful tool for discovering patterns and structures in the unlabeled dataset, which we have seen is available in medical and smart home environments. Due to the high number of ADLs performed by the residents of the smart homes, it is not feasible to enumerate every activity. Even in hospital settings, EHR and other medical records are available in such a way that opens the avenue to exploit clustering algorithms to learn patient data automatically. However, such clustering techniques do not give good accuracy compared to supervised learning methods. Another important aspect to consider is that if the information is collected over a more extended period, it will have diversified features, making clustering difficult if several clusters are defined a priori.
Reinforcement Learning
Reinforcement is a sub-category of artificial intelligence which trains the model by rewarding desired behaviour and punishing undesired behaviour. The unique characteristic of Reinforcement Learning (RL) is that it solves the problem by allowing the agent to collaborate and interact with the environment. The agent takes the most appropriate action according to its learning. If the action is correct, the agent rewards itself and vice versa. In this way, the agent keeps on learning without the interference of any human being. The process of reinforcement learning is illustrated in Fig. 6.
RL has been used to solve various real-life problems [181]. Reinforcement learning shows promising results when applied in assesment [104, 188] and detection [182,183,184,185,186,187] of cognitive health. It can sustain change for a more extended time. Some important RL methods are discussed below:
Authors in [104] described a Deep Recurrent Q Learning-based Reinforcement Model (DRQLRM) based on the reinforcement learning process. This model is designed to investigate the presence of Alzheimer’s in individuals. In this model, a retraining stage has been added in which a recurrent neural network is used to reduce the overfitting. In the learning stage, the reinforcement approach identified the unknown pattern. Sensor data is collected from the CASA smart home of 400 persons. The dataset comprised Activities of Daily Living (ADL), such as cooking, bathing, and toileting. DRQLRM shows promising results with 98% Accuracy and 12% MSE. Authors in [182] presented an LSTM-based reinforcement learning technique to detect early dementia and guided the patients who have dementia to perform ADL. LSTM model is used to infer the current goal of the person by observing previous normal ADL patterns to infer the current state. The authors then used a situ-learning agent that helped individuals to take the right action, thus preventing adverse events while guiding individuals through the task sequence that led to the goal state. In addition, a naive agent is also used to resolve the uncertainty older adults with early-stage dementia might experience. The accuracy achieved by this algorithm is 90.1%.
Authors in [188] performed individual and population-level behaviour analysis from time series sensor data. They developed a novel algorithm-Resident Relative Entropy-Inverse Reinforcement Learning (RRE-IRL) — to analyse a single smart home resident or a group of residents from time series sensor data. By employing this method, an individual’s behavioural routine preferences are learned. It then analysed an individual’s daily routines and observed that routine behavioural preferences change over time. Furthermore, the behaviour preferences are used by a random forest classifier to predict a resident’s cognitive health diagnosis, with an accuracy of 0.84. One limitation of this technique is the participant sample size. Their analyses are based on large data collected from smart homes over many days. However, data for only eight participants are considered.
Authors in [183] proposed an interpretable multimodal deep reinforcement learning model. It helps in the diagnosis of AD. In the first step, the compressed-sensing MRI image is reconstructed using an interpretable deep reinforcement learning model. Then, the resultant MRI is fed into the CNN to generate a pixel-level disease probability risk map (DPM) of the whole brain for AD. Finally, an attention-based deep CNN is used to classify Alzheimer’s patients. The algorithm was tested on ADNI, AIBL, and NACC datasets and obtained an accuracy of 99.6% for ANDI.
A reinforcement learning framework is designed in [184] which uses an agent trained on clinical transcripts and sketches a disease-specific lexical probability distribution. Therefore, it detects MCI by dialogue with the patient in a minimum number of conversations. In [186] a reinforcement learning-based technique showed promising results. Multi-agent reinforcement learning is discussed in [185] which detected anatomical landmarks from MRI images using a novel communicative multi-agent reinforcement learning (C-MARL). Authors in [189] Alzheimer’s disease progression is presented by combining differential equations and reinforcement learning with a domain knowledge model. The researchers use deep Q-Network [187] for landmark detection in 3D medical scans in a single and multi-agent. Another automatic detection of anatomical landmarks is discussed by [186], which robustly localised target landmarks in medical scans and is also helpful in further investigation of diseases like Alzheimer’s. Table 6 presents the summary of reinforcement learning studies for cognitive health assessment.
Critical Analysis: Reinforcement learning has achieved tremendous success in the past years despite its complex nature, notably in games but unfortunately, in the medical context, particularly in cognitive health assessment applications, it has not appropriately benefited from this technique. In most AI-based health systems, the sequential nature of decisions is not considered. Instead, the decisions exclusively rely on the current state of patients. RL offers an attractive alternative to such systems, considering the immediate effect of treatment and the long-term benefit to the patient. Besides the potential of RL to make a revolution in the medical field, there are a few obstacles that have to be removed in order to apply RL algorithms in health assessment. RL algorithm typically learns by its action, but guesswork has no room in the medical field. Moreover, RL algorithms should be designed to learn from existing data.
Natural Language Processing Techniques
Natural Language Processing (NLP) is the ability of computers to understand the verbal and written communication of human beings [190, 191]. NLP includes speech recognition, sentiment analysis, and optical character recognition [192]. NLP works by transferring data into a format understandable by NLP algorithms. NLP has been used in assesment [193] and detection [105, 194,195,196,197,198,199,200] of cognitive health. In these applications, NLP techniques are applied to the natural language speech or text, followed by some ML techniques, i.e. Supervised Learning, Unsupervised Learning, and Deep Learning for classification purposes. Figure 7 is an illustration of the main features of NLP.
Authors in [198], speech recordings are transcribed, and linguistic and acoustic variables are extracted through NLP and automated speech analysis. These linguistic and acoustic variables help find a cognitively impaired person who faces difficulty in finding the correct word while speaking. NLP-extracted variables include lexical, semantic, and syntactic aspects of the recording. Acoustic variables include sound wave properties, speech rate, and several pauses. Spearman’s correlation is used to find the correlations between the NLP technique and clinically captured speech characteristics.
Speech disfluency is defined as any pause or abruption which occurs during fluent speech. Cognitively impaired people have a short vocabulary, which leads to speech disfluencies. Therefore speech disfluency can detect cognitively impaired people. Authors in [194] asked Patients to give spontaneous answers to the questions asked by an expert by recalling two short back and white films. Acoustic parameters in a patient’s speech signals are noted both manually and automatically. The ML technique then uses these features to classify participants into healthy and unhealthy classes. These features are then used to classify the patients in MCI and healthy controls classes with an F1-score of 78.8%. Acoustic parameters used are hesitation ratio, speech tempo, length and number of silent and filled pauses, and length of utterance.
NLP technique for detecting the mild cognitively impaired person is presented in [195]. Mann–Whitney U-test is used to select the most relevant features from speech. For classification into MCI and healthy control class, ML algorithms used are using K-nearest neighbours (K-NN), Support Vector Machines, Multilayer Perceptron (MLP), and a CNN. Classification Error Rate (CER) has been used to evaluate the results. Early-stage dementia and mild cognitive impairment are important to identify, and authors in [197] used Linguistic features with a Transformer-based deep learning model to detect whether the individual has dementia. This model can detect linguistic deficits with much accuracy.
Yi et al. [196] indicated that NLP models have the potential to extract lifestyle information from clinical notes. This lifestyle information can include extreme diet, physical activity, sleep deprivation, and substance abuse. Various ML models are trained and evaluated on NLP exacted data. The bagging, random forest, KNN, and random forest showed promising results among these models.
A method to detect preclinical stages of dementia is discussed in [201] where 96 people are asked to describe a complex picture, a typical working day, and recall a last remembered dream. Linguistic features of spontaneous speech transcribed and analysed by NLP techniques showed significant differences between controls and pathological states. These features include computing rhythmic, acoustic, lexical, morpho-syntactic, and syntactic features, and participants are categorised as healthy or cognitively impaired. The statistical analysis is performed by the non-parametric Kruskal-Wallis test and Mann–Whitney U-test.
Psychological disorders could affect cognitive health. Therefore detection of psychological disorders is also as important in the cognitive health assessment scenario. Psychological disorders like bipolar disorder, obsessive-compulsive disorder, and schizophrenia can be detected from speech input using the model discussed in [193]. This paper used an ASGD Weight-Dropped long short-term memory (AWD-LSTM) language model for psychological disease diagnosis.
In [202] progress notes and discharge summaries are parsed using NLP techniques. This paper has used NLP with Electronic Health Record data. Machine learning techniques logistic regression, multilayer 37 perceptrons, and random forest are used to classify impaired and healthy persons using the clinical terms extracted by NLP. Random forest method showed the best performance. The limitation of this technique is that it can only give good results if the input dataset size is large. Authors in [203] discussed the NLP approach based on clinical notes. This approach extracts lifestyle exposures and intervention strategies to predict Alzheimer’s disease. MetaMap tool maps biomedical texts to standard medical concepts using the Unified Medical Language System (UMLS). Validity of results is done by comparing the proposed algorithm with data captured independently by clinicians. Authors in [204] discussed the use of NLP. Features are extracted from electronic medical records with the help of the NLP algorithm, and then the classification algorithm identifies whether a person has dementia or not. Classification algorithms discussed in this paper are gradient boosted models, neural networks, lasso, and ridge regression. Authors in [205] developed a system for identifying MCI from clinical text without screening or other structured diagnostic information. NLP and Least absolute shrinkage and selection operator logistic regression approach (LASSO) were used to detect MCI. Chatting and immediate formation of new sentences can detect a person with cognitive impairment, as discussed by [199]. They proposed an automatic detection of cognitive impairment through chatbot dialogue. NLP algorithms were used to generate the sentences for a chatbot. Authors in [206] fully automated MCI detection technique is proposed based on multilingual word embeddings to create multilingual information units. Researchers trained an ANN using the spectrogram of the audio signal to detect Alzheimer’s in [105]. To distinguish between MCI and early-stage Alzheimer’s, patients are asked to perform tasks such as countdown, picture description, Scene description, and Semantic fluency (animals) [207]. The patients’ responses are recorded and analysed using NLP algorithms. Spontaneous speech again showed an excellent tool to be used in Alzheimer’s detection [208]. The authors proposed an NLP-based model for detecting early Alzheimer’s through spontaneous speech datasets in 2020. A recent technique [200] proposed an ensemble classifier based on four classifiers: audio, language, dysfluency, and interactivity. This system works with two modules proactive listener and ensemble AD detector. Authors in [209] explored the lexical performance through spontaneous speech to detect Alzheimer’s.
The relevance of acoustic features of spontaneous speech for cognitive impairment detection is shown in [210]. Here the authors applied machine learning methods for classification, which showed promising results. Authors of [105] claimed that they produced a fully automated audio file processing without manual feature extraction. Therefore they introduced a new direction of cognitive health assessment through NLP. Authors in [211] an algorithm is proposed for predicting probable Alzheimer’s disease using linguistic deficits and biomarkers with the help of NLP and machine learning. Authors in [212] automated analysis of Semantic Verbal Fluency (SVF) tests method is discussed for detection of MCI. Table 7 presents the summary of NLP studies for cognitive health assessment.
Critical Analysis: The rise of big data analytics has proven NLP to be a potential approach in clinical settings to assess patients’ cognitive health. Electronic Health Records, spontaneous speech, discharge summaries, and progress notes contain much important information about cognitive abilities. NLP is helping the healthcare industry to make the best use of the myriad insights hidden in unstructured data. NLP attempts to be the heart of cognitive health assessment when applied to lifestyle exposures and intervention strategies of adults to gather and extract features that can then be fed into a classification model to discriminate between healthy and diseased persons. However, NLP has to address various challenges when applied to the cognitive health assessment task. The main challenge of applying NLP is to make such data available/recorded, which is not easy in the traditional clinical setting. Another big challenge is that understanding clinical language often requires deep subject matter knowledge and integrating many separate pieces of context. Most of the words have multiple meanings. Phrasing Ambiguities and Language differences also play a role in the generalisation of the NLP model.
Computer Vision/Image Processing
Recent advances in computer vision show vibrant performance for clinical applications. When combined with deep learning techniques, computer vision techniques further enhance a system’s ability to assess [213, 214] and detect [106, 215,216,217,218] cognitive diseases correctly. A few computer vision/image processing-based techniques are discussed here, which help identify patients with mild or severe cognitive impairment. Figure 8 illustrates how computer vision can be used for facial expression.
Authors in [213] claimed that facial emotions could be helpful in predicting the cognitive state of a person. They captured facial expressions of different emotions, and Montreal Cognitive assessment was used to evaluate all participants. This technique helped to identify patients at an early stage of cognitive impairment and other mature stages. Montreal Cognitive Assessment criteria are used to classify the individuals based on the extracted features. A similar concept of facial expression utilisation is used in [215]. The proposed model combined computer vision and Alexnet to identify cognitively impaired subjects. Its results are tested on bench-marking databases like JAFFE, KDEF, CK+, and FER2013. In [216] computer vision-based technique, i.e. optical flow, is used to detect patients having Alzheimer’s. This system worked on a video that contained a Patient and automatically collect statistical data about the handclapping of a patient. This statistical data includes the frequency of clapping, the extent of clapping, and direction change. Results showed that these statistics are important if applied to disease diagnosis.
Diffusion Tensor Imaging (DTI) is a new imaging modality giving more information than anatomical MRI; therefore, it is used to identify cognitively impaired persons more accurately [217]. Researchers extracted biomarkers from DTI and sMRI, which are then used to classify individuals in AD and MCI classes of cognitive disorders. Authors in [106] showed that dementia and gait disorders are highly correlated. Therefore, they acquired gait data using a Kinect sensor, human pose estimation was performed, and gait features were analysed to identify the persons with cognitive disorders. Authors in [218] proposed a technique that used inertial sensors to detect gait phases in cognitively impaired persons. In [214], a system “CogniLearn” is proposed for assessing physical exercises. The head-Toes-Knees-Shoulders (HTKS) task is widely used as a cognitive evaluation tool for children and young adults. These physical exercises are designed to monitor the cognitive health of an individual. CogniLearn inspected physical activities by using a deep learning architecture and suggested action which is to be taken to address any health issue. Users of this system performed HTKS games, and CogniLearn automatically captured the motion and performed analysis to provide detailed evaluations by recognising activities with the help of computer vision and deep learning techniques. This system is evaluated by a self-created dataset with 15 subjects and four variations of the HTKS tasks. CNN, feNet, and Caffe are used to evaluate the captured data. Table 8 presents the summary of computer vision-based studies for cognitive health assessment.
Critical Analysis: The Healthcare industry has recognised the immense potential of computer vision in powering technology. CV and DL provide a revolutionary mechanism with the ultimate level of automation capabilities, one that only requires some fine-tuning to handle large datasets to work on its own successfully. The biggest advantage of computer vision techniques in cognitive health assessment is that CV techniques perform judgment in real-time monitoring regarding the cognitive health of a person by capturing their daily routine activities (gait, clapping, physical exercises, etc.). CV algorithms diagnose a change in the cognitive health of a person accurately and timely, thus eliminating the need to go to a hospital, especially to get oneself checked up. Moreover, these techniques can also detect patterns in medical images, assisting a physician. Therefore we can conclude that the world of computer vision changed with the evolution of deep learning. It has proved to be a very effective tool capable of dramatically improving cognitive disease diagnosis.
Artificial intelligence is likely to transform healthcare in the near future. However, a few concerns need to be addressed in this regard. For AI systems to work in healthcare, these systems must gain the confidence of healthcare professionals and patients. To gain trust, the transparency of AI systems should be enhanced. In the future, policies should ensure that healthcare professionals should be involved in the design and implementation of such systems and introduce new regulations overseeing the transparent design, validation, deployment, and certification of systems leveraging artificial intelligence.
There is a need to convert the black boxes of typical deep learning models into transparent boxes with predictable results. Therefore, researchers are now focusing on such models which allow predictions generated by the neural network model as interpretable.
Data Acquisition Channels
Acquiring data is the most fundamental process in the automation process. Various modes and techniques have been used for data gathering or acquisition, which is further used for cognitive health assessment [219]. Data gathering is crucial in MCI detection. Objective assessment of patients in the most accurate environment and gathering physical responses in different scenarios play a vital role in understanding the patient’s mental health. Many researchers have suggested different tools to gather accurate data [220].
Sensor-based Data Acquisition
Sensor-based data acquisition entails gathering data through inertial, physiological, and environmental sensors. They are further categorised concerning the use and placement of the sensors, e.g. wearable sensor-based data acquisition, smartphone-based data acquisition, and smart home-based data acquisition.
-
Wearable sensor-based acquisition:
Wearable sensors most commonly used in the domain of cognitive health are ECG, magnetometer, accelerometer, and gyroscope sensors, along with temperature, light, and pressure sensors. EEG, ECG, and other sensors to monitor physiological biomarkers are also commonly used. The authors in [221] proposed a real-time context-aware automated cognitive health assessment model that uses wearable physiological sensors comprising electrodermal and photoplethysmography sensors and physical sensors like accelerometers alongside ambient sensors to capture data of participants in a free-living environment. These sensors gather the participants’ physiological health indicators and hand and postural gestures. Chen et al. [222] proposes a design of a home-based assessment model using an iPhone, apple watch, A Digital Assessment app, a Beddit sleep monitoring app, and an iPad to gather multimodal sensor stream data. Sensor data alongside participant device usage, history, calls, and message information is collected and presented as data to different algorithms to analyse patterns and behaviour related to CI. Bringas et al. [223] is another work that utilises time series data provided by accelerometers to assess the movement patterns of participants to classify them according to the CI stage. Commercially available wearable devices have been used to acquire different physiological readings. Zephyr BioHarness34 for ECG monitoring [224] and Development Kit5 for Electrodermal activity [224], CorSense finger-worn device as a heart rate variability monitor [225]. Wearable devices attached to shoes that include inertial Micro-Electro-Mechanical System (MEMS) sensors have been fixed under the heel of the shoes to collect gait information of patients and assess their cognitive health [226]. Shimmer3 IMU [224, 227] is commonly used to capture a patient’s physical activity readings. The SensorFootV2, a wearable inertial sensor part of the SmartWalk system, is placed on the feet of patients as well to gather gait-related data [228].
-
Smart home-based sensors:
With the growing IoT industry, smart homes have become a promising tool to predict, diagnose and manage MCI, AD, and dementia. Data is gathered from the surroundings of a smart home resident. With the help of multiple sensors, physical, psychological, and physiological health can be observed, which can help predict health problems timely and effective. Researchers have used the smart home setting to suggest effective models for estimating cognitive health. A combination of different sensors like flow sensors, infrared sensors, pressure sensors, and switch sensors have been proposed by authors in [229]. These sensors have been employed in a room-like setting, and daily behaviour data is gathered using a Wireless Sensor Network (WSN) for pattern identification. This data is then used to distinguish standard patterns from abnormal patterns. Authors in [230] have proposed a fully integrated smart home-based prediction model for Alzheimer’s Disease symptom detection. The authors gathered 2yr time series statistics on ten behavioural features via varying modalities. Mobility and activity data were gathered via activity sensors coupled with self-reported questionnaires data gathered to evaluate cognition and mood. Authors in [72] proposed a new approach CA-SHR that uses data gathered from sensor stream data from Simple Daily Life Activities (SADL) and ADL to develop and evaluate the efficacy of early detection and diagnosis of MCI, dementia, and AD. In one such research [231], the authors used temperature and humidity sensors, gas sensors, fluid detection sensors, pressure-sensitive mats, and LDR photoresistance sensors to collect raw data. This sensor data enabled continuous behaviour monitoring and also helped alert caregivers in case of a fall or danger of a gas or water tap left open. Infrared motion and proximity sensors capture patient movement data within the smart home. A motion sensor gives a good insight into walking speed and ADL patterns. Depth cameras like Microsoft Kinect are also used in smart homes to obtain images and videos of residents while performing ADL. To monitor sleep activities, sleep sensors and infrared sensors are installed in beds [232]. Other sensors called contextual sensors are mounted in devices like stoves, sinks, refrigerators, doors, and medicine boxes capture consumption data [233]
-
Smart phone-based data acquisition:
The potential of smartphones for cognitive health-related data acquisition is by far the most. Embedded smartphone sensors not only gather the patient’s environmental and physical data but also can obtain physiological data and prompt the patients for mood and cognitive assessment at regular intervals to obtain a comprehensive picture of the patient’s mental health. Smartphones can gather high-dimensional datasets from a larger population with utmost ease. Several researchers have used smartphones as a primary tool to obtain raw data regarding MCI, dementia, and AD sufferers. An android-based smartphone app was also used in [234] to gather touch and visual stimulus responses to track CI. The app was able to automate decision support for Cognitive Assessment. Similarly, in [235], the author has also used a smartphone-based assessment model to gather information from participants. The author has conducted a Cognitive Linguistic Assessment via the smartphone app and a Behaviour Assessment using a FitBit-like wearable device. Gait assessment is also conducted to gather data that is then analysed via different methods like AI and statistics. Lauraitis et al. [234] is another research where the authors have developed an android app to assess a patient’s cognitive function by performing three tasks that involves touching the screen at the correct speed and with precision. The inability to complete the tasks is marked as a symptom of cognitive decline.
Datasets and Electronic Health Record (EHR)
EHR are digital patient records that contain patients’ demographic and medical history, treatment plans, immunisation records, allergies, laboratory and test results, and hospital treatment history, including billing and payment information. These datasets can be shared across different providers. Some of these datasets are released in the open public data repository that researchers can use for analysis and research. Some of the most famous datasets that have contributed to research in the field of cognitive health are the famous Alzheimer’s Disease Neuroimaging Initiative (ADNI), Open Access Series of Imaging Studies (OASIS), positron emission tomography (PET), Magnetic Resonance Imaging (MRI), Computerised Topography (CT), Electroencephalography (EEG), Magnetoencephalography (MEG) and Cerebrospinal fluid (CSF) biomarkers [52, 54]. Questionnaire response data and data from the ADL datasets have also been used for disease diagnosis, progression, and management. Authors in [139] used MRI images, standard cognitive tests, demographic information like age, gender, ethnicity, and education, and some genetic information collected at the initial patient visit. This data was passed onto a Deep Learning (DL) model that predicts the risk of a patient diagnosed with MCI developing AD in 3 years. Kalafatis et al. [236] employed a combination of computerised self-administered assessment tools like MoCA and ACE-iii to develop an Integrated Cognitive Assessment (ICA) tool. The data gathered from these assessments are then used to detect CI using Artificial Intelligence techniques. Javed et al. [72] is a smart home-based solution that uses a popular sensor stream data containing Activities of Daily Living (ADL) and applied ML algorithms to predict and analyse CI. A detailed summary of different datasets commonly used to access data regarding the cognitive health of patients has been discussed in “Cognitive Health Assessment Datasets” section.
Clinical Data and Questionnaires
A clinical evaluation is a clinician’s subjective report of a physical exam conducted in a health clinic. This information combines patient history, a physical checkup, and other disease-specific investigations and assessments. The gold standard for CHA has been in-clinic evaluation, self-administered questionnaires, and interview-administered questionnaires by health practitioners. Some of these have complied in Table 3. Other assessment tests for mobility include the “Timed Up and Go Test,” the Functional Reach Test, the Berg Balance Scale, the Arm Curl Test, handgrip strength, and the Montreal Cognitive Assessment [237]. Interview-based questionnaires and activities further help gather speech and response data. Using Natural Language Processing (NLP) technique on this data has allowed early identification of linguistic signs of MCI in the elderly [198, 201].
Virtual Reality-based Data Acquisition
Augmented Reality (AR) and Virtual Reality (VR) have allowed for more accurate data acquisition by allowing real-time communication and computation. Computer simulations replace a patient’s sensory world with a virtual environment that changes in real-time in response to a patient’s physical movement and activities. Although it is relatively costly, this is closer to reality experience and gives unbiased responses, hence the most accurate data. The authors in [238] designed an exciting data collection mechanism incorporating a multimethod approach using Virtual Reality (VR) and gaits kinematic analysis to gather participant data. CAVE (Cave Automated Virtual Environment) gave participants a realistic experience while performing ADL (Activities of Daily Life). This helped make the participants feel more comfortable, helping them obtain accurate responses and behaviour. VR was also employed by [239] whereby data was gathered using a virtual supermarket. This model gathered information based on executive function tasks and improved the accuracy of diagnosis by evaluating in a real-life-like situation. Therapists have used VR cognitive training games to enable patients to perform their daily activities with considerable ease. In [240], two subsystems have been proposed, one for the patient and one for the therapist. The patient must complete assigned tasks in a virtual game environment, and the therapist controls the training sessions and monitors the patient’s behaviour. A similar Virtual Reality (VR)-Based Environmental Enrichment program was proposed by [241]; the authors created virtual environments of famous landmarks to assess different cognitive domains like memory, attention, orientation, recognition, and executive functioning. The scope of VR for CH is not limited to prediction and diagnosis; in fact, VR is commonly used by the therapist for training and assistance for CI. It helps a patient train so that it can deal with life-like scenarios without actually making mistakes in daily life that could otherwise be disastrous.
Cognitive Health Assessment Datasets
Data quality is the foundation of all AI techniques. Good quality data enhances the learning process of the model. Several modes of data acquisition have been discussed in “Data Acquisition Channels” section. One of these techniques is publicly available datasets. The choice of a dataset depends on the accuracy of the dataset, its completeness, reliability, relevance, and timeliness. Datasets relevant to CHA need to be relevant to disease progression, the symptoms, the changes in a patient’s daily activities, speech and language behaviour, and responses. Several studies were analysed to observe different datasets used for cognitive health assessment.
NeuroImaging Datasets
Among the most famous NeuroImaging datasets are the Alzheimer’s Disease NeuroImaging Initiative (ADNI) dataset [242,243,244,245,246,247], Australian Imaging biomarkers and lifestyle study (AIBL) [248,249,250,251,252], Open Access Series of Imaging Studies (OASIS-3) [122, 253,254,255,256], Internet Brain Segmentation Repository (IBSR) [257,258,259,260], and Medical Image Computing and Computer Assisted Intervention Brain Tumor Segmentation (MICCAI BraTs) [261,262,263]. Image-based datasets include images of MRI, PET and FMRI scans. ADNI, AIBL and OASIS-3 datasets include demographic, clinical and biomarkers data of participants in the dataset thus giving a much more comprehensive outcome. Feature extraction from images can give accurate outcomes if the dataset is considerably large, therefore ADNI, AIBL and OASIS-3 are valuable datasets consisting a large number of MRI, PET, FMRI and clinical data records of patients. Most researchers have successfully used them to obtain the most accurate disease prediction and diagnosis results.
Activities of Daily Living (ADL) Datasets
The second most common type of dataset that researchers have used to predict and diagnose MCI, AD, and dementia is the ADL dataset. An ADL dataset consists of data on the daily activities of different subjects obtained from IMUs (Inertial Measurement Units) made of accelerometers, gyroscopes, and magnetometers. The most commonly used were the VanKasteren dataset [264,265,266,267], Center of Advanced Studies in Adaptive Systems (CASAS) dataset [268,269,270,271], and UCI HAR dataset [272,273,274]. UniMiB SHAR dataset [275, 276], DU MD dataset [277], and CAD datasets [278] are not as standard but were found in some researches too. The VanKasteren and the CASAS datasets are extensive datasets that acquire ADL information via smart homes and test beds that acquire ADL of healthy individuals over a prolonged time. These datasets have been valuable in differentiating CI patients from their healthy counterparts.
Speech and Language Datasets
Another set of data that was commonly analysed to differentiate patients with MCI, AD, and dementia from normal, healthy individuals was the speech and language data. It has been observed that speech and language alterations are among the first signs of cognitive decline [201]. Therefore many researchers have analysed the speech and language of a possible MCI patient for early detection. Some of the most commonly used datasets were the DimentiaBank dataset [197, 210, 279,280,281], LibriSpeech dataset [279, 282, 283], Alzheimer’s Dementia Recognition through Spontaneous Speech (ADReSS) dataset [197, 279, 280], and Trinity College Dublin Speaker Ageing (TCDSA) dataset [280]. These datasets contain audio recordings of CI patients and healthy individuals, which have been used by researchers to analyse and detect CI in individuals.
Other Datasets
Several researchers have used data from different cohort studies to compare behaviour or MCI vs. normal healthy individuals. These longitudinal studies were gathered from hospitals and patients from different countries. Including the cohort, study datasets were not possible since they varied significantly based on location. A few research studies included Magnetoencephalography (MEG) dataset [284, 285]. MEG is a medical exam of the brain’s ability to produce magnetic impulses. The analysis is based upon the findings obtained from the test to be able to distinguish healthy from MCI patients. Another commonly used dataset is the Uniform Dataset (UDS) [286,287,288], which analyses cognitive health. This dataset contains questionnaire responses of different individuals to questions related to the cognitive assessment domains mentioned in Fig. 3.
Table9 is a summary of the most commonly used datasets for CHA.
Challenges and Open Issues and Future Directions
This section discusses some of the challenges and open issues in using AI/ML for cognitive health, along with possible solutions.
Security and Privacy Preservation
AI can play a significant role in assessing/predicting patients’ cognitive health. However, AI algorithms must be trained with a significant amount of data to uncover the patterns existing in the datasets. This data includes sensitive and private patient information, which must be preserved at any cost. Malicious users may get hold of private and sensitive data of patients and misuse/tamper with them. Hence, training the AI algorithms in a privacy-preserving manner is a significant challenge [289, 290].
Federated learning (FL) is a recent development in AI/ML that offloads the ML model to the data source. The ML model will be trained at the source of the data rather than the central cloud as in a conventional ML setup. Only the parameters from the local devices will be sent across to the central cloud for global training, hence preserving the privacy of the sensitive information related to the cognitive health of the patients. In this way, FL can be used to strengthen the privacy preservation of the data related to the cognitive health of the patients [291]. Blockchain is a disruptive technology that can be used to tamper-proof cognitive health data.
Blockchain can also prevent malicious users from entering the network; thereby, it can help maintain the confidentially and integrity of cognitive health data [292].
Multi-hospital Collaboration
One of the challenges in training the AI/ML algorithms on cognitive healthcare data is the lack of sufficient data. AI/ML algorithms need a large volume of data to train [293]. This problem may arise due to a limited number of patients in a single hospital. To address this problem, AI/ML model can be trained on the data collated from multiple hospitals. However, the hospitals may be hesitant to work collaboratively by sharing their patients’ private and personal information as it may lead to the issue of privacy preservation.
FL, in which the raw data need not be transferred to the central storage, can be a possible solution to solve this issue [294, 295]. Through FL, the ML model can be distributed across individual hospitals to get trained on cognitive health data in a privacy-preserving manner. The model updates will then be aggregated from the individual hospitals to train the global ML model. In this way, the FL can solve the multi-hospital collaboration issue of lack of adequate data [296].
Optimisation of Repetitive Tasks During Cognitive Rehabilitation
Even after surgery/treatment, the patients may still not recover some of the lost cognitive skills due to brain injury. Cognitive rehabilitation therapy (CRT) is suggested to the patients to restore their cognitive functions completely. CRT is a set of therapies that medical practitioners use to restore/improve cognitive functions in patients who experience injuries in the brain due to stroke, traumatic brain injury, or some other reasons [297]. There are several kinds of CRT techniques that medical practitioners can follow. However, a technique that can be successful for one patient may not work for others. AI/ML algorithms can help identify the right kind of therapy for a particular patient. Based on the recommendations of AI/ML algorithms, the therapists can give appropriate exercises to the patients [298]. However, most of these tasks are repetitive and do not need to be addressed by skilled manpower who can work on other important tasks during that time. These repetitive tasks can be assigned to robots that can execute the required therapies on the patients to give the therapy to the patients [299, 300].
Quality and Dimensionality of the Data
One of the challenges in using IoMT or allied technologies in cognitive healthcare to monitor the patients and extract the data through sensors is the quality of the data generated. The data generated from these devices may have some noise or missing values due to malfunctioning of the devices or network connectivity problems. Another challenge is that all the attributes may not be required to train the ML model as some attributes may not impact the class label. The huge dimensionality of the data may increase the complexity of the ML model and also may have a negative impact on the performance of the model. To overcome these issues, thorough pre-processing techniques must be executed before training the ML model on the data. Some popular dimensionality reduction techniques, such as linear discriminant analysis, principal component analysis, regression, and clustering, can be applied to the dataset before training the ML models [301, 302].
Patients Not Reacting Well to the Medicines
Every patient has a different metabolism/condition or may have allergies to a certain drug combination. Hence, a medicine that works well for one patient with a cognitive disease may not work well for other patients with the same condition. Hence giving the right dosage and drugs to patients with cognitive health problems is a significant challenge. Digital twins are a recent development where a digital replica of a patient can be created and tested on the drug combinations and dosages of medicines. Based on the reaction of the digital twin to the drugs, precision medicine can be given to the patients [303].
Interpretability/Justification
The machine/deep learning models are often black boxes in nature, i.e. we may never know how and why the ML/AI models have made a particular prediction [25, 304, 305]. This lack of explanation/interpretation/justification on why a particular decision is made hinders medical practitioners from adopting AI/ML models for diagnosing patients with cognitive health problems [15]. Even though the accuracy of AI/ML models is increased significantly, these models have several misclassified instances. Hence, doctors and other healthcare professionals hesitate to diagnose patients with AI/ML-based models. To overcome this problem, Explainable AI (XAI) can be used. XAI can explain/justify why the AI model gives a particular prediction or what factors made the model give a particular decision [306, 307].
Conclusion
This paper summarises the state-of-the-art on the use of AI for the assessment of cognitive/mental health. We explored different AI approaches for this purpose. Our findings suggest that among the supervised approaches, SVM, ensemble methods and neural networks like CNN, AlexNet, LeNet5, MLP, and GoogLeNet, seem to have yielded the best accuracies. Among the unsupervised approaches, K-means, K-Nearest Neighbour, Clustering, AutoEncoders and Deep Q-network reinforcement learning seemed to be the most prevalent, resulting in good accuracies for the prediction of mental health problems. NLP techniques and computer vision have also been very promising in detecting cognitive decline at early stages. A look at some of the datasets that have contributed to the research in the domain shows that NeuroImaging datasets and ADL datasets have gained focus in many researches and have helped train the AI models in predicting and diagnosing cognitive health problems effectively. This survey also helps bring to light the current challenges that are being faced to improve the accuracy of prediction and diagnosis so that future researchers are able to identify the research gaps better.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sarraf S, Desouza DD, Anderson JA, Saverino C. MCADNNet: recognizing stages of cognitive impairment through efficient convolutional fMRI and MRI neural network topology models. IEEE Access. 2019;7:155584–600.
Khatun S, Morshed BI, Bidelman GM. A single-channel EEG-based approach to detect mild cognitive impairment via speech-evoked brain responses. IEEE Trans Neural Syst Rehabil Eng. 2019;27(5):1063–70.
Javed AR, Shahzad F, ur Rehman S, Zikria YB, Razzak I, Jalil Z, et al. Future smart cities requirements, emerging technologies, applications, challenges, and future aspects. Cities. 2022;129:103794.
Ramzan F, Khan MUG, Rehmat A, Iqbal S, Saba T, Rehman A, et al. A deep learning approach for automated diagnosis and multi-class classification of Alzheimer’s disease stages using resting-state fMRI and residual neural networks. J Med Syst. 2020;44(2):1–16.
Association A. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–87.
Chaudhary S, Kumaran SS, Kaloiya G, Goyal V, Sagar R, Kalaivani M, et al. Domain specific cognitive impairment in Parkinson’s patients with mild cognitive impairment. J Clin Neurosci. 2020;75:99–105.
Rahman MA. Gaussian process in computational biology: covariance functions for transcriptomics [phd]. University of Sheffield; 2018. https://etheses.whiterose.ac.uk/19460/.
Rakib AB, Rumky EA, Ashraf AJ, Hillas MM, Rahman MA. Mental healthcare chatbot using sequence-to-sequence learning and BiLSTM. In: Mahmud M, Kaiser MS, Vassanelli S, Dai Q, Zhong N, editors. Brain Informatics. Cham: Springer International Publishing; 2021. p. 378–87.
Islam N, et al. Towards machine learning based intrusion detection in IoT networks. Comput Mater Contin. 2021;69(2):1801–21.
Farhin F, Kaiser MS, Mahmud M. Secured smart healthcare system: blockchain and bayesian inference based approach. In: Proceedings of TCCE. 2021. p. 455–65.
Ahmed S, et al. Artificial intelligence and machine learning for ensuring security in smart cities. In: Data-driven Mining, Learning and Analytics for Secured Smart Cities. Springer; 2021. p. 23–47.
Zaman S, et al. Security threats and artificial intelligence based countermeasures for internet of things networks: a comprehensive survey. IEEE Access. 2021;9:94668–90.
Noor MBT, Zenia NZ, Kaiser MS, Mamun SA, Mahmud M. Application of deep learning in detecting neurological disorders from magnetic resonance images: a survey on the detection of Alzheimer’s disease, Parkinson’s disease and schizophrenia. Brain Inform. 2020;7(1):1–21.
Ghosh T, Al Banna MH, Rahman MS, Kaiser MS, Mahmud M, Hosen AS, et al. Artificial intelligence and internet of things in screening and management of autism spectrum disorder. Sustain Cities Soc. 2021;74: 103189.
Biswas M, Kaiser MS, Mahmud M, Al Mamun S, Hossain M, Rahman MA, et al. An XAI based autism detection: the context behind the detection. In: Proceedings of Brain Informatics. 2021. p. 448–59.
Wadhera T, Mahmud M. Computing hierarchical complexity of the brain from electroencephalogram signals: a graph convolutional network-based approach. In: Proceeding of IJCNN. 2022. p. 1–6.
Wadhera T, Mahmud M. Influences of social learning in individual perception and decision making in people with autism: a computational approach. In: Proceedings of Brain Informatics. 2022. p. 50–61.
Wadhera T, Mahmud M. Brain networks in autism spectrum disorder, epilepsy and their relationship: a machine learning approach. In: Artificial Intelligence in Healthcare: Recent Applications and Developments. Springer; 2022. p. 125–42.
Wadhera T, Mahmud M. Brain functional network topology in autism spectrum disorder: a novel weighted hierarchical complexity metric for electroencephalogram. IEEE J Biomed Health Inform. 2023:1–8.
Sumi AI, et al. fASSERT: a fuzzy assistive system for children with autism using internet of things. In: Proceedings of Brain Inform. 2018. p. 403–12.
Akhund NU, et al. ADEPTNESS: Alzheimer’s disease patient management system using pervasive sensors-early prototype and preliminary results. In: Proceedings of Brain Informatics. 2018. p. 413–22.
Al Banna M, Ghosh T, Taher KA, Kaiser MS, Mahmud M, et al. A monitoring system for patients of autism spectrum disorder using artificial intelligence. In: Proceedings of Brain Informatics. 2020. p. 251-62.
Jesmin S, Kaiser MS, Mahmud M. Artificial and internet of healthcare things based Alzheimer care during COVID 19. In: Proceedings of Brain Informatics. 2020. p. 263–74.
Ahmed S, Hossain M, Nur SB, Shamim Kaiser M, Mahmud M, et al. Toward machine learning-based psychological assessment of autism spectrum disorders in school and community. In: Proceedings of TEHI. 2022. p. 139–49.
Mahmud M, Kaiser MS, Rahman MA, Wadhera T, Brown DJ, Shopland N, et al. Towards explainable and privacy-preserving artificial intelligence for personalisation in autism spectrum disorder. In: Universal Access in Human-Computer Interaction. User and Context Diversity: 16th International Conference, UAHCI 2022, Held as Part of the 24th HCI International Conference, HCII 2022, Virtual Event, June 26–July 1, 2022, Proceedings, Part II. Springer; 2022. p. 356–70.
Nahiduzzaman M, et al. Machine learning based early fall detection for elderly people with neurological disorder using multimodal data fusion. In: Proceedings of Brain Informatics. 2020. p. 204–14.
Biswas M, et al. Indoor navigation support system for patients with neurodegenerative diseases. In: Proceedings of Brain Informatics. 2021. p. 411–22.
Sadik R, Reza ML, Al Noman A, Al Mamun S, Kaiser MS, Rahman MA. COVID-19 pandemic: a comparative prediction using machine learning. International Journal of Automation, Artificial Intelligence and Machine Learning. 2020;1(1):1–16.
Mahmud M, Kaiser MS. Machine learning in fighting pandemics: a COVID-19 case study. In: COVID-19: prediction, decision-making, and its impacts. Springer; 2021. p. 77–81.
Kumar S, et al. Forecasting major impacts of COVID-19 pandemic on country-driven sectors: challenges, lessons, and future roadmap. Pers Ubiquitous Comput. 2021:1–24.
Bhapkar HR, et al. Rough sets in COVID-19 to predict symptomatic cases. In: COVID-19: Prediction, Decision-Making, and its Impacts. Springer; 2021. p. 57–68.
Satu MS, et al. Short-term prediction of COVID-19 cases using machine learning models. Appl Sci. 2021;11(9):4266.
Prakash N, et al. Deep transfer learning for COVID-19 detection and infection localization with superpixel based segmentation. Sustain Cities Soc. 2021;75: 103252.
AlArjani A, et al. Application of mathematical modeling in prediction of COVID-19 transmission dynamics. Arab J Sci Eng. 2022:1-24.
Paul A, et al. Inverted bell-curve-based ensemble of deep learning models for detection of COVID-19 from chest X-rays. Neural Comput Appl. 2022:1-15.
Mahmud M, Kaiser MS, Rahman MM, Rahman MA, Shabut A, Al-Mamun S, et al. A brain-inspired trust management model to assure security in a cloud based IoT framework for neuroscience applications. Cogn Comput. 2018;10(5):864–73.
Mahmud M, Kaiser MS, Hussain A, Vassanelli S. Applications of deep learning and reinforcement learning to biological data. IEEE Trans Neural Netw Learn Syst. 2018;29(6):2063–79.
Mahmud M, Kaiser MS, McGinnity TM, Hussain A. Deep learning in mining biological data. Cogn Comput. 2021;13(1):1–33.
Nasrin F, Ahmed NI, Rahman MA. Auditory attention state decoding for the quiet and hypothetical environment: a comparison between bLSTM and SVM. In: Kaiser MS, Bandyopadhyay A, Mahmud M, Ray K, editors. Proceedings of TCCE. Advances in Intelligent Systems and Computing. Singapore: Springer; 2021. p. 291–301.
Rahman MA, Brown DJ, Mahmud M, Shopland N, Haym N, Sumich A, et al. Biofeedback towards machine learning driven self-guided virtual reality exposure therapy based on arousal state detection from multimodal data. In: Proceedings of BI2022. 2022. p. 1–12.
Farhin F, Kaiser MS, Mahmud M. Towards secured service provisioning for the internet of healthcare things. In: Proceedings of AICT. 2020. p. 1–6.
Kaiser MS, et al. 6G access network for intelligent internet of healthcare things: opportunity, challenges, and research directions. In: Proceedings TCCE. 2021. p. 317–28.
Biswas M, et al. ACCU3RATE: a mobile health application rating scale based on user reviews. PLoS ONE. 2021;16(12): e0258050.
Rabby G, et al. A flexible keyphrase extraction technique for academic literature. Procedia Comput Sci. 2018;135:553–63.
Rabby G, Azad S, Mahmud M, Zamli KZ, Rahman MM. Teket: a tree-based unsupervised keyphrase extraction technique. Cogn Comput. 2020;12(4):811–33.
Adiba FI, Islam T, Kaiser MS, Mahmud M, Rahman MA. Effect of corpora on classification of fake news using Naive Bayes classifier. International Journal of Automation, Artificial Intelligence and Machine Learning. 2020;1(1):80–92. Number: 1. https://researchlakejournals.com/index.php/AAIML/article/view/45.
Das S, Yasmin MR, Arefin M, Taher KA, Uddin MN, Rahman MA. Mixed Bangla-english spoken digit classification using convolutional neural network. In: Kasabov N, Iftekharuddin K, Zhong N, editors. Kaiser MS. Mahmud M. Applied Intelligence and Informatics. Communications in Computer and Information Science. Cham: Springer International Publishing; 2021. p. 371–83.
Nawar A, Toma NT, Al Mamun S, Kaiser MS, Mahmud M, Rahman MA. Cross-content recommendation between movie and book using machine learning. In: 2021 IEEE 15th International Conference on Application of Information and Communication Technologies (AICT). 2021. p. 1–6.
Rahman MA, Brown DJ, Shopland N, Burton A, Mahmud M. explainable multimodal machine learning for engagement analysis by continuous performance test. In: Stephanidis C, editor. Antona M. Universal Access in Human-Computer Interaction. User and Context Diversity. Lecture Notes in Computer Science. Cham: Springer International Publishing; 2022. p. 386–99.
Rahman MA, Brown DJ, Shopland N, Harris MC, Turabee ZB, Heym N, et al. Towards machine learning driven self-guided virtual reality exposure therapy based on arousal state detection from multimodal data. In: Mahmud M, He J, Vassanelli S, van Zundert A, Zhong N, et al. editors. Brain Informatics. Cham: Springer International Publishing; 2022. p. 195–209.
Mahmud M, Kaiser MS, Rahman MA. Towards explainable and privacy-preserving artificial intelligence for personalisation in autism spectrum disorder. In: Stephanidis C, Antona M, editor. Universal Access in Human-Computer Interaction. User and Context Diversity. Lecture Notes in Computer Science. Cham: Springer International Publishing; 2022. p. 356–70.
Zhao X, Ang CKE, Acharya UR, Cheong KH. Application of artificial intelligence techniques for the detection of Alzheimer’s disease using structural MRI images. Biocybernetics and Biomedical Engineering. 2021;41(2):456–73.
Graham SA, Lee EE, Jeste DV, Van Patten R, Twamley EW, Nebeker C, et al. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: a conceptual review. Psychiatry Res. 2020;284: 112732.
Dashwood M, Churchhouse G, Young M, Kuruvilla T. Artificial intelligence as an aid to diagnosing dementia: an overview. Prog Neurol Psychiatry. 2021;25(3):42–7.
Fabrizio C, Termine A, Caltagirone C, Sancesario G. Artificial intelligence for Alzheimer’s disease: promise or challenge? Diagnostics. 2021;11(8):1473.
Tăuţan AM, Ionescu B, Santarnecchi E. Artificial intelligence in neurodegenerative diseases: a review of available tools with a focus on machine learning techniques. Artif Intell Med. 2021;117: 102081.
de la Fuente Garcia S, Ritchie CW, Luz S. Artificial intelligence, speech, and language processing approaches to monitoring Alzheimer’s disease: a systematic review. J Alzheimers Dis. 2020;78(4):1547–74.
Öhman F, Hassenstab J, Berron D, Schöll M, Papp KV. Current advances in digital cognitive assessment for preclinical Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2021;13(1): e12217.
Piau A, Wild K, Mattek N, Kaye J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: systematic review. J Med Internet Res. 2019;21(8):e12785.
Aslam RW, Bates V, Dundar Y, Hounsome J, Richardson M, Krishan A, et al. A systematic review of the diagnostic accuracy of automated tests for cognitive impairment. Int J Geriatr Psychiatry. 2018;33(4):561–75.
Tsang G, Xie X, Zhou SM. Harnessing the power of machine learning in dementia informatics research: Issues, opportunities, and challenges. IEEE Rev Biomed Eng. 2019;13:113–29.
Thabtah F, Peebles D, Retzler J, Hathurusingha C. Dementia medical screening using mobile applications: a systematic review with a new mapping model. J Biomed Inform. 2020;111: 103573.
Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. The humanistic and economic burden of Alzheimer’s Disease. Neurol Ther. 2022:1-27.
Abd Razak M, Ahmad N, Chan Y, Kasim NM, Yusof M, Ghani MA, et al. Validity of screening tools for dementia and mild cognitive impairment among the elderly in primary health care: a systematic review. Public Health. 2019;169:84–92.
Harvey PD. Domains of cognition and their assessment. Dialogues Clin Neurosci. 2022.
Zhuang L, Yang Y, Gao J. Cognitive assessment tools for mild cognitive impairment screening. J Neurol. 2021;268(5):1615–22.
Campos-Vasquez F, Valdez-Murrugarra N, Soto-Tarazona A, Camacho-Caballero K, Rodriguez-Cuba M, Parodi J, et al. Concordance between the mini-mental state examination, short portable mental status questionnaire and montreal cognitive assessment tests for screening for cognitive impairment in older adults. Adv Gerontol. 2021;11(3):312–6.
Beishon LC, Batterham AP, Quinn TJ, Nelson CP, Panerai RB, Robinson T, et al. Addenbrooke’s Cognitive Examination III (ACE-III) and mini-ACE for the detection of Dementia and mild cognitive impairment. Cochrane Database Syst Rev. 2019;12(12).
Aldabbas H, Albashish D, Khatatneh K, Amin R. An architecture of IoT-aware healthcare smart system by leveraging machine learning. Int Arab J Inf Technol. 2022;19:160–72.
Maksimović M, Vujović V, Periśić BA, custom Internet of Things healthcare system. In: 10th Iberian Conference on Information Systems and Technologies (CISTI), vol. 2015. IEEE; 2015. p. 1–6.
Sarker IH, Khan AI, Abushark YB, Alsolami F. Internet of things (IoT) security intelligence: a comprehensive overview, machine learning solutions and research directions. Mob Netw Appl. 2022:1–17.
Javed AR, Fahad LG, Farhan AA, Abbas S, Srivastava G, Parizi RM, et al. Automated cognitive health assessment in smart homes using machine learning. Sustain Cities Soc. 2021;65: 102572.
Javed AR, Sarwar MU, Khan S, Iwendi C, Mittal M, Kumar N. Analyzing the effectiveness and contribution of each axis of tri-axial accelerometer sensor for accurate activity recognition. Sensors. 2020;20(8):2216.
Ahmad W, Rasool A, Javed AR, Baker T, Jalil Z. Cyber security in iot-based cloud computing: a comprehensive survey. Electronics. 2021;11(1):16.
Shabbir M, Shabbir A, Iwendi C, Javed AR, Rizwan M, Herencsar N, et al. Enhancing security of health information using modular encryption standard in mobile cloud computing. IEEE Access. 2021;9:8820–34.
Asif S, Ambreen M, Muhammad Z, ur Rahman H, Iqbal S. Cloud computing in healthcare-investigation of threats, vulnerabilities, future challenges and counter measure. LC International Journal of STEM (ISSN: 2708-7123). 2022;3(1):63–74.
Yadav S, Kaushik A, Sharma S. Simplify the difficult: artificial intelligence and cloud computing in healthcare. IoT and Cloud Computing for Societal Good. 2022:101–24.
Liu J, Guo H, Fadlullah ZM, Kato N. Energy consumption minimization for FiWi enhanced LTE-A HetNets with UE connection constraint. IEEE Commun Mag. 2016;54(11):56–62.
Chen B, Wan J, Celesti A, Li D, Abbas H, Zhang Q. Edge computing in IoT-based manufacturing. IEEE Commun Mag. 2018;56(9):103–9.
Alrazgan M. Internet of medical things and edge computing for improving healthcare in smart cities. Math Probl Eng. 2022;2022.
Alqahtani A, Alsubai S, Sha M, Khan MA, Alhaisoni M, Naqvi SR. Automated white blood cell disease recognition using lightweight deep learning. Comput Syst Sci Eng. 2023;46(1):107–23.
Pelekoudas-Oikonomou F, Zachos G, Papaioannou M, de Ree M, Ribeiro JC, Mantas G, et al. Blockchain-based security mechanisms for IoMT Edge networks in IoMT-based healthcare monitoring systems. Sensors. 2022;22(7):2449.
Hajiheydari N, Delgosha MS, Olya H. Scepticism and resistance to IoMT in healthcare: application of behavioural reasoning theory with configurational perspective. Technol Forecast Soc Chang. 2021;169: 120807.
Ajagbe SA, Awotunde JB, Adesina AO, Achimugu P, Kumar TA. Internet of Medical Things (IoMT): applications, challenges, and prospects in a data-driven technology. Intelligent Healthcare. 2022:299–319.
Dhiyya AJA. Architecture of IoMT in healthcare. The Internet of Medical Things (IoMT) Healthcare Transformation. 2022:161–72.
Belle A, Thiagarajan R, Soroushmehr S, Navidi F, Beard DA, Najarian K. Big data analytics in healthcare. Biomed Res Int. 2015;2015.
Karatas M, Eriskin L, Deveci M, Pamucar D, Garg H. Big Data for Healthcare Industry 4.0: applications, challenges and future perspectives. Expert Syst Appl. 2022:116912.
Pramanik PKD, Pal S, Mukhopadhyay M. Healthcare big data: a comprehensive overview. Research Anthology on Big Data Analytics, Architectures, and Applications. 2022:119–47.
Kaiser MS, Zenia N, Tabassum F, Mamun SA, Rahman MA, Islam MS, et al. 6G access network for intelligent internet of healthcare things: opportunity, challenges, and research directions. In: Proceedings of International Conference on Trends in Computational and Cognitive Engineering: Proceedings of TCCE 2020. Springer; 2020. p. 317–28.
Hu J, Liang W, Hosam O, Hsieh MY, Su X. 5GSS: A framework for 5G-secure-smart healthcare monitoring. Connect Sci. 2022;34(1):139–61.
Prakash V, Garg L, Camilleri L, Curmi J, Camilleri D. 5G in Healthcare: features, advantages, limitations, and applications. In: Implementing Data Analytics and Architectures for Next Generation Wireless Communications. IGI Global. 2022. p. 51–68.
Alshammari N, Sarker MNI, Kamruzzaman M, Alruwaili M, Alanazi SA, Raihan ML, et al. Technology-driven 5G enabled e-healthcare system during COVID-19 pandemic. IET Commun. 2022;16(5):449–63.
Nazir S, Ali Y, Ullah N, García-Magariño I. Internet of things for healthcare using effects of mobile computing: a systematic literature review. Wirel Commun Mob Comput. 2019;2019.
Hamza A, Khan MA, Alhaisoni M, Al Hejaili A, Shaban KA, Alsubai S, et al. D2BOF-COVIDNet: A Framework of Deep Bayesian Optimization and Fusion-Assisted Optimal Deep Features for COVID-19 Classification Using Chest X-ray and MRI Scans. Diagnostics. 2023;13(1):101.
Fakhoury M, Fritz M, Sleiman SF. Behavioral paradigms for assessing cognitive functions in the chronic social defeat stress model of depression. In: Translational Research Methods for Major Depressive Disorder. Springer; 2022. p. 147–64.
Rahman MA, Brown DJ, Shopland N, Harris MC, Turabee ZB, Heym N, et al. Towards machine learning driven self-guided virtual reality exposure therapy based on arousal state detection from multimodal data. In: Brain Informatics: 15th International Conference, BI 2022, Padua, Italy, July 15–17, 2022, Proceedings. Springer; 2022. p. 195–209.
Rahman MA, Brown DJ, Mahmud M, Shopland N, Haym N, Sumich A, et al. Biofeedback towards machine learning driven self-guided virtual reality exposure therapy based on arousal state detection from multimodal data. Brain Informatics. 2023.
Srivastava K, Das RC, Chaudhury S. Virtual reality applications in mental health: Challenges and perspectives. Ind Psychiatry J. 2014;23(2):83.
Nevin L, Editors PM. Advancing the beneficial use of machine learning in health care and medicine: toward a community understanding. PLoS Medicine. 2018;15(11):e1002708.
Raghupathi W, Raghupathi V. Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;2(1):1–10.
Graham S, Depp C, Lee EE, Nebeker C, Tu X, Kim HC, et al. Artificial intelligence for mental health and mental illnesses: an overview. Curr Psychiatry Rep. 2019;21(11):1–18.
Esteva A, Robicquet A, Ramsundar B, Kuleshov V, DePristo M, Chou K, et al. A guide to deep learning in healthcare. Nat Med. 2019;25(1):24–9.
Bzdok D, Krzywinski M, Altman N. Machine learning: supervised methods. Nat Methods. 2018;15(1):5.
Dhanusha C, Kumar AS. Deep recurrent Q reinforcement learning model to predict the alzheimer disease using smart home sensor data. In: IOP Conference Series: Materials Science and Engineering. vol. 1074. 2021. p. 012014.
Bertini F, Allevi D, Lutero G, Calzà L, Montesi D. An automatic Alzheimer’s disease classifier based on spontaneous spoken English. Computer Speech & Language. 2022;72: 101298.
Kondragunta J, Hirtz G, Gait parameter estimation of elderly people using 3D human pose estimation in early detection of dementia. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE. 2020. p. 5798–801.
Cook DJ, Strickland M, Schmitter-Edgecombe M. Detecting smartwatch-based behavior change in response to a multi-domain brain health intervention. ACM Trans Comput Healthc (HEALTH). 2022;3(3):1–18.
Sharma A, Kaur S, Memon N, Fathima AJ, Ray S, Bhatt MW. Alzheimer’s patients detection using support vector machine (SVM) with quantitative analysis. Neuroscience Informatics. 2021;1(3): 100012.
Dawadi PN, Cook DJ, Schmitter-Edgecombe M. Automated cognitive health assessment from smart home-based behavior data. IEEE J Biomed Health Inform. 2015;20(4):1188–94.
Dawadi PN, Cook DJ, Schmitter-Edgecombe M. Automated cognitive health assessment using smart home monitoring of complex tasks. IEEE Trans Syst Man Cybern Syst. 2013;43(6):1302–13.
Shahparian N, Yazdi M, Khosravi MR. Alzheimer disease diagnosis from fMRI images based on latent low rank features and support vector machine (SVM). Curr Signal Transduct Ther. 2021;16(2):171–7.
López M, Ramírez J, Górriz J, Salas-Gonzalez D, Alvarez I, Segovia F, et al. Automatic tool for Alzheimer’s disease diagnosis using PCA and Bayesian classification rules. Electron Lett. 2009;45(8):389–91.
Liu S, Song Y, Cai W, Pujol S, Kikinis R, Wang X, et al. Multifold Bayesian kernelization in Alzheimer’s diagnosis. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer; 2013. p. 303–10.
Laske C, Leyhe T, Stransky E, Hoffmann N, Fallgatter AJ, Dietzsch J. Identification of a blood-based biomarker panel for classification of Alzheimer’s disease. Int J Neuropsychopharmacol. 2011;14(9):1147–55.
Afzal S, Javed M, Maqsood M, Aadil F, Rho S, Mehmood I. A segmentation-less efficient Alzheimer detection approach using hybrid image features. In: Handbook of Multimedia Information Security: Techniques and Applications. Springer; 2019. p. 421–9.
Lopez M, Ramirez J, Gorriz J, Salas-Gonzalez D, Alvarez I, Segovia F, et al. Neurological image classification for the Alzheimer’s Disease diagnosis using Kernel PCA and Support Vector Machines. In: 2009 IEEE Nuclear Science Symposium Conference Record (NSS/MIC). IEEE. 2009. p. 2486–9.
Dukart J, Mueller K, Barthel H, Villringer A, Sabri O, Schroeter ML, et al. Meta-analysis based SVM classification enables accurate detection of Alzheimer’s disease across different clinical centers using FDG-PET and MRI. Psychiatry Res Neuroimaging. 2013;212(3):230–6.
Hackmack K, Paul F, Weygandt M, Allefeld C, Haynes JD, Initiative ADN, et al. Multi-scale classification of disease using structural MRI and wavelet transform. Neuroimage. 2012;62(1):48–58.
Johnson P, Vandewater L, Wilson W, Maruff P, Savage G, Graham P, et al. Genetic algorithm with logistic regression for prediction of progression to Alzheimer’s disease. BMC Bioinf. 2014;15(16):1–14.
Mazzocco T, Hussain A. Novel logistic regression models to aid the diagnosis of dementia. Expert Syst Appl. 2012;39(3):3356–61.
Brasil Filho AT, Pinheiro PR, Coelho AL. Towards the early diagnosis of Alzheimer’s disease via a multicriteria classification model. In: International Conference on Evolutionary Multi-criterion Optimization. Springer; 2009. p. 393–406.
Yagis E, Citi L, Diciotti S, Marzi C, Atnafu SW, De Herrera AGS. 3D convolutional neural networks for diagnosis of Alzheimer’s disease via structural MRI. In: 2020 IEEE 33rd International Symposium on Computer-Based Medical Systems (CBMS). IEEE; 2020. p. 65–70.
Ahmad RH, Fakhoury M, Lawand N. Electromagnetic field in Alzheimer’s disease: a literature review of recent preclinical and clinical studies. Curr Alzheimer Res. 2020;17(11):1001–12.
Stamate D, Smith R, Tsygancov R, Vorobev R, Langham J, Stahl D, et al. Applying deep learning to predicting dementia and mild cognitive impairment. In: IFIP International Conference on Artificial Intelligence Applications and Innovations. Springer; 2020. p. 308–19.
Catá Villá M. Feature selection methods for predicting pre-clinical stage in Alzheirmer’s Disease [B.S. thesis]. Universitat Politècnica de Catalunya. 2014.
Alsubai S, Khan HU, Alqahtani A, Sha M, Abbas S, Mohammad UG. Ensemble deep learning for brain tumor detection. Front Comp Sci. 2022.
Gerardin E, Chételat G, Chupin M, Cuingnet R, Desgranges B, Kim HS, et al. Multidimensional classification of hippocampal shape features discriminates Alzheimer’s disease and mild cognitive impairment from normal aging. Neuroimage. 2009;47(4):1476–86.
Zhang YD, Wang S, Dong Z. Classification of Alzheimer disease based on structural magnetic resonance imaging by kernel support vector machine decision tree. Prog Electromagn Res. 2014;144:171–84.
Padilla P, López M, Górriz JM, Ramirez J, Salas-Gonzalez D, Alvarez I. NMF-SVM based CAD tool applied to functional brain images for the diagnosis of Alzheimer’s disease. IEEE Trans Med Imaging. 2011;31(2):207–16.
Ferreira LK, Rondina JM, Kubo R, Ono CR, Leite CC, Smid J, et al. Support vector machine-based classification of neuroimages in Alzheimer’s disease: direct comparison of FDG-PET, rCBF-SPECT and MRI data acquired from the same individuals. Braz J Psychiatry. 2017;40:181–91.
Zhao M, Chan RH, Tang P, Chow TW, Wong SW. Trace ratio linear discriminant analysis for medical diagnosis: a case study of dementia. IEEE Signal Process Lett. 2013;20(5):431–4.
Meng X, Wei Q, Meng L, Liu J, Wu Y, Liu W. Feature fusion and detection in Alzheimer’s disease using a novel genetic multi-kernel SVM based on MRI imaging and gene data. Genes. 2022;13(5):837.
Ahamed F, Shahrestani S, Cheung H. Internet of things and machine learning for healthy ageing: identifying the early signs of dementia. Sensors. 2020;20(21):6031.
Kashefpoor M, Rabbani H, Barekatain M. Supervised dictionary learning of EEG signals for mild cognitive impairment diagnosis. Biomed Signal Process Control. 2019;53:101559.
Dahmen J, Cook DJ. Indirectly supervised anomaly detection of clinically meaningful health events from smart home data. ACM Trans Intell Syst Technol (TIST). 2021;12(2):1–18.
Alberdi A, Aztiria A, Basarab A, Cook DJ. Using smart offices to predict occupational stress. Int J Ind Ergon. 2018;67:13–26.
Su CF, Fu LC, Chien YW, Li TY. Activity recognition system for dementia in smart homes based on wearable sensor data. In: 2018 IEEE Symposium Series on Computational Intelligence (SSCI). IEEE; 2018. p. 463–9.
Cook DJ, Schmitter-Edgecombe M. Fusing ambient and mobile sensor features into a behaviorome for predicting clinical health scores. IEEE Access. 2021;9:65033–43.
Spasov S, Passamonti L, Duggento A, Lio P, Toschi N, Initiative ADN, et al. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to Alzheimer’s disease. Neuroimage. 2019;189:276–87.
Choi H, Jin KH, Initiative ADN, et al. Predicting cognitive decline with deep learning of brain metabolism and amyloid imaging. Behav Brain Res. 2018;344:103–9.
Habuza T, Zaki N, Statsenko Y, Alnajjar F, Elyassami S. Deep learning for predicting cognitive gap as a reliable biomarker of dementia. medRxiv. 2021.
Kherchouche A, Ben-Ahmed O, Guillevin C, Tremblais B, Julian A, Fernandez-Maloigne C, et al. Attention-guided neural network for early Dementia detection using MRS data. Comput Med Imaging Graph. 2022:102074.
Sarraf S, Tofighi G. Classification of Alzheimer’s disease using fMRI data and deep learning convolutional neural networks. arXiv:1603.08631 [Preprint] . 2016. Available from: http://arxiv.org/abs/1603.08631.
Fulton LV, Dolezel D, Harrop J, Yan Y, Fulton CP. Classification of Alzheimer’s disease with and without imagery using gradient boosted machines and ResNet-50. Brain Sci. 2019;9(9):212.
Farooq A, Anwar S, Awais M, Rehman S. A deep CNN based multi-class classification of Alzheimer’s disease using MRI. In: 2017 IEEE International Conference on Imaging systems and techniques (IST). IEEE; 2017. p. 1–6.
Miotto R, Wang F, Wang S, Jiang X, Dudley JT. Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2018;19(6):1236–46.
De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67(8):949–56.
Rodrigues PM, Freitas D, Teixeir JP. Alzheimer electroencephalogram temporal events detection by K-means. Procedia Technol. 2012;5:859–64.
Wang T, Cook DJ. Multi-person activity recognition in continuously monitored smart homes. IEEE Trans Emerg Top Comput. 2021.
Fisher CK, Smith AM, Walsh JR. Machine learning for comprehensive forecasting of Alzheimer’s Disease progression. Sci Rep. 2019;9(1):1–14.
Papakostas GA, Savio A, Graña M, Kaburlasos VG. A lattice computing approach to Alzheimer’s disease computer assisted diagnosis based on MRI data. Neurocomputing. 2015;150:37–42.
Wang Z, Tang Z, Zhu Y, Pettigrew C, Soldan A, Gross A, et al. AD risk score for the early phases of disease based on unsupervised machine learning. Alzheimers Dement. 2020;16(11):1524–33.
Fahad LG, Tahir SF. Activity recognition and anomaly detection in smart homes. Neurocomputing. 2021;423:362–72.
Escudero J, Zajicek JP, Ifeachor E, Early detection and characterization of Alzheimer’s disease in clinical scenarios using Bioprofile concepts and K-means. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE; 2011. p. 6470–3.
Escudero J, Ifeachor E, Zajicek JP, Initiative ADN, et al. Bioprofile analysis: a new approach for the analysis of biomedical data in Alzheimer’s disease. J Alzheimers Dis. 2012;32(4):997–1010.
Tosto G, Monsell SE, Hawes SE, Bruno G, Mayeux R. Progression of extrapyramidal signs in Alzheimer’s disease: clinical and neuropathological correlates. J Alzheimers Dis. 2016;49(4):1085–93.
Racine AM, Nicholas CR, Clark LR, Koscik RL, Okonkwo OC, Hillmer AT, et al. O1-01-03: Alzheimer’s disease biomarker-based clusters predict amyloid accumulation and cognitive decline in a preclinical cohort: findings from the Wisconsin registry for Alzheimer’s prevention (WRAP). Alzheimers Dement. 2015;11(7S_Part_3):P123-5.
Armstrong RA, Wood L. The identification of pathological subtypes of Alzheimer’s disease using cluster analysis. Acta Neuropathol. 1994;88(1):60–6.
Fouladvand S, Mielke MM, Vassilaki M, Sauver JS, Petersen RC, Sohn S. Deep learning prediction of mild cognitive impairment using electronic health records. In: 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM). IEEE; 2019. p. 799-806.
Suk HI, Lee SW, Shen D. Latent feature representation with stacked auto-encoder for AD/MCI diagnosis. Brain Struct Funct. 2015;220(2):841–59.
Jin S, Zou P, Han Y, Jiang J, Unsupervised detection of individual atrophy in Alzheimer’s disease. In: 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE; 2021:2647–50.
Baydargil HB, Park JS, Kang DY. Anomaly analysis of Alzheimer’s disease in PET images using an unsupervised adversarial deep learning model. Appl Sci. 2021;11(5):2187.
Varghese T, Sheela KR, Mathuranath P, Singh A. Evaluation of different stages of Alzheimer’s disease using unsupervised clustering techniques and voxel based morphometry. In: 2012 World Congress on Information and Communication Technologies. IEEE; 2012. p. 953–8.
Horn JF, Habert MO, Kas A, Malek Z, Maksud P, Lacomblez L, et al. Differential automatic diagnosis between Alzheimer’s disease and frontotemporal dementia based on perfusion SPECT images. Artif Intell Med. 2009;47(2):147–58.
Price CC, Tanner JJ, Schmalfuss IM, Brumback B, Heilman KM, Libon DJ. Dissociating statistically-determined Alzheimer’s disease/vascular dementia neuropsychological syndromes using white and gray neuroradiological parameters. J Alzheimers Dis. 2015;48(3):833–47.
Hwang J, Kim CM, Jeon S, Lee JM, Hong YJ, Roh JH, et al. Prediction of Alzheimer’s disease pathophysiology based on cortical thickness patterns. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;2:58–67.
Gamberger D, Ženko B, Mitelpunkt A, Shachar N, Lavrač N. Clusters of male and female Alzheimer’s disease patients in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. Brain Informatics. 2016;3(3):169–79.
Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, et al. Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology. 2014;83(21):1936–44.
McCurry SM, Logsdon RG, Teri L, Gibbons LE, Kukull WA, Bowen JD, et al. Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J Geriatr Psychiatry Neurol. 1999;12(2):53–9.
Bi X, Li S, Xiao B, Li Y, Wang G, Ma X. Computer aided Alzheimer’s disease diagnosis by an unsupervised deep learning technology. Neurocomputing. 2020;392:296–304.
Suk HI, Shen D. Deep learning-based feature representation for AD/MCI classification. In: International conference on medical image computing and computer-assisted intervention. Springer; 2013. p. 583–90.
Hosseini-Asl E, Gimel’farb G, El-Baz A. Alzheimer’s disease diagnostics by a deeply supervised adaptable 3D convolutional network. arXiv:1607.00556 [Preprint]. 2016. Available from: http://arxiv.org/abs/1607.00556.
Liu S, Liu S, Cai W, Pujol S, Kikinis R, Feng D, Early diagnosis of Alzheimer’s disease with deep learning. In: 2014 IEEE 11th International Symposium on Biomedical Imaging (ISBI). IEEE; 2014. p. 1015–8.
Suk HI, Lee SW, Shen D, Initiative ADN, et al. Hierarchical feature representation and multimodal fusion with deep learning for AD/MCI diagnosis. Neuroimage. 2014;101:569–82.
Suk HI, Wee CY, Lee SW, Shen D. State-space model with deep learning for functional dynamics estimation in resting-state fMRI. Neuroimage. 2016;129:292–307.
Han C, Rundo L, Murao K, Noguchi T, Shimahara Y, Milacski ZÁ, et al. MADGAN: Unsupervised medical anomaly detection GAN using multiple adjacent brain MRI slice reconstruction. BMC Bioinformatics. 2021;22(2):1–20.
Han C, Rundo L, Murao K, Milacski ZÁ, Umemoto K, Sala E, et al. GAN-based multiple adjacent brain MRI slice reconstruction for unsupervised Alzheimer’s disease diagnosis. In: International Meeting on Computational Intelligence Methods for Bioinformatics and Biostatistics. Springer; 2019. p. 44–54.
Cabreza JN, Solano GA, Ojeda SA, Munar V. Anomaly detection for Alzheimer’s disease in brain MRIs via unsupervised generative adversarial learning. In: 2022 International Conference on Artificial Intelligence in Information and Communication (ICAIIC). IEEE; 2022. p. 1–5.
Sajjad M, Ramzan F, Khan MUG, Rehman A, Kolivand M, Fati SM, et al. Deep convolutional generative adversarial network for Alzheimer’s disease classification using positron emission tomography (PET) and synthetic data augmentation. Microsc Res Tech. 2021;84(12):3023–34.
Pei S, Guan J. Classifying cognitive normal and early mild cognitive impairment of Alzheimer’s disease by applying restricted Boltzmann machine to fMRI data. Curr Bioinform. 2021;16(2):252–60.
Rathnayaka M, Watawala W, Manamendra M, Silva S, Kasthurirathna D, Jayalath T. Cognitive rehabilitation based personalized solution for Dementia patients using reinforcement learning. In: 2021 IEEE International Systems Conference (SysCon). IEEE; 2021. p. 1–6.
Oyeleke RO, Chang CK, Margrett J. Situation-centered goal reinforcement of activities of daily living in smart home environments. Expert Syst. 2020;37(1): e12487.
Zhang Q, Du Q, Liu G. A whole-process interpretable and multi-modal deep reinforcement learning for diagnosis and analysis of Alzheimer’s disease. J Neural Eng. 2021;18(6): 066032.
Tang F, Lin K, Uchendu I, Dodge HH, Zhou J. Improving mild cognitive impairment prediction via reinforcement learning and dialogue simulation. arXiv:1802.06428 [Preprint]. 2018. Available from: http://arxiv.org/abs/1802.06428.
Leroy G, Rueckert D, Alansary A. Communicative reinforcement learning agents for landmark detection in brain images. In: Machine Learning in Clinical Neuroimaging and Radiogenomics in Neuro-oncology. Springer; 2020. p. 177–86.
Alansary A, Oktay O, Li Y, Le Folgoc L, Hou B, Vaillant G, et al. Evaluating reinforcement learning agents for anatomical landmark detection. Med Image Anal. 2019;53:156–64.
Kundeti SR, Parmar D, Sanin A, Gautam D. Landmark detection in 3D medical images using reinforcement learning. In: 2020 IEEE International Conference on Cloud Computing in Emerging Markets (CCEM). IEEE; 2020. p. 42–6.
Lin B, Cook DJ. Analyzing sensor-based individual and population behavior patterns via inverse reinforcement learning. Sensors. 2020;20(18):5207.
Saboo K, Choudhary A, Cao Y, Worrell G, Jones D, Iyer R. Reinforcement learning based disease progression model for Alzheimer’s disease. Adv Neural Inf Process Syst. 2021;34:20903–15.
Hirschberg J, Manning CD. Advances in natural language processing. Science. 2015;349(6245):261–6.
Zhang T, Schoene AM, Ji S, Ananiadou S. Natural language processing applied to mental illness detection: a narrative review. NPJ digital medicine. 2022;5(1):1–13.
Moreira LB, Namen AA. A hybrid data mining model for diagnosis of patients with clinical suspicion of dementia. Comput Methods Programs Biomed. 2018;165:139–49.
Kamra V, Kumar P, Mohammadian M. Natural language processing enabled cognitive disease prediction model for varied medical records implemented over ML techniques. In: 2021 3rd International Conference on Signal Processing and Communication (ICPSC). IEEE; 2021. p. 494–8.
Tóth L, Hoffmann I, Gosztolya G, Vincze V, Szatlóczki G, Bánréti Z, et al. A speech recognition-based solution for the automatic detection of mild cognitive impairment from spontaneous speech. Curr Alzheimer Res. 2018;15(2):130–8.
Lopez-de Ipiña K, Martinez-de Lizarduy U, Calvo PM, Beitia B, García-Melero J, Ecay-Torres M, et al. Analysis of disfluencies for automatic detection of mild cognitive impartment: a deep learning approach. In: International Conference and Workshop on Bioinspired Intelligence (IWOBI), vol. 2017. IEEE; 2017. p. 1–4.
Yi Y, Shen Z, Bompelli A, Yu F, Wang Y, Zhang R. Natural language processing methods to extract lifestyle exposures for Alzheimer’s disease from clinical notes. In: 2020 IEEE International Conference on Healthcare Informatics (ICHI). IEEE; 2020. p. 1-2.
Saltz P, Lin SY, Cheng SC, Si D. Dementia detection using transformer-based deep learning and natural language processing models. In: 2021 IEEE 9th International Conference on Healthcare Informatics (ICHI). IEEE; 2021. p. 509–10.
Yeung A, Iaboni A, Rochon E, Lavoie M, Santiago C, Yancheva M, et al. Correlating natural language processing and automated speech analysis with clinician assessment to quantify speech-language changes in mild cognitive impairment and Alzheimer’s dementia. Alzheimer’s research & therapy. 2021;13(1):1–10.
de Arriba-Pérez F, García-Méndez S, González-Castaño FJ, Costa-Montenegro E. Automatic detection of cognitive impairment in elderly people using an entertainment chatbot with Natural Language Processing capabilities. J Ambient Intell Humaniz Comput. 2022:1–16.
Li Y, Lai C, Lala D, Inoue K, Kawahara T. Alzheimer’s Dementia detection through spontaneous dialogue with proactive robotic listeners. In: HRI; 2022. p. 875–9.
Beltrami D, Gagliardi G, Rossini Favretti R, Ghidoni E, Tamburini F, Calzà L. Speech analysis by natural language processing techniques: a possible tool for very early detection of cognitive decline? Frontiers in aging neuroscience. 2018;10:369.
Hussein KI, Chan L, Van Vleck T, Beers K, Mindt MR, Wolf M, et al. Natural language processing to identify patients with cognitive impairment. medRxiv. 2022.
Zhou X, Wang Y, Sohn S, Therneau TM, Liu H, Knopman DS. Automatic extraction and assessment of lifestyle exposures for Alzheimer’s disease using natural language processing. Int J Med Informatics. 2019;130: 103943.
Maclagan LC, Abdalla M, Harris DA, Chen B, Candido E, Swartz RH, et al. Using natural language processing to identify signs and symptoms of dementia and cognitive impairment in primary care electronic medical records (EMR). Alzheimer’s & Dementia. 2021;17: e054091.
Penfold RB, Carrell DS, Cronkite DJ, Pabiniak C, Dodd T, Glass AM, et al. Development of a machine learning model to predict mild cognitive impairment using natural language processing in the absence of screening. BMC Med Inform Decis Mak. 2022;22(1):1–13.
Fraser KC, Fors KL, Kokkinakis D. Multilingual word embeddings for the assessment of narrative speech in mild cognitive impairment. Comput Speech Lang. 2019;53:121–39.
König A, Satt A, Sorin A, Hoory R, Toledo-Ronen O, Derreumaux A, et al. Automatic speech analysis for the assessment of patients with predementia and Alzheimer’s disease. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2015;1(1):112–24.
Liu N, Luo K, Yuan Z, Chen Y. A transfer learning method for detecting Alzheimer’s disease based on speech and natural language processing. Front Public Health. 2022;10.
Liu N, Yuan Z. Spontaneous language analysis in Alzheimer’s disease: evaluation of natural language processing technique for analyzing lexical performance. J Shanghai Jiaotong Univ (Sci). 2022;27(2):160–7.
Haider F, De La Fuente S, Luz S. An assessment of paralinguistic acoustic features for detection of Alzheimer’s dementia in spontaneous speech. IEEE J Sel Top Sign Proces. 2019;14(2):272–81.
Orimaye SO, Wong JS, Golden KJ, Wong CP, Soyiri IN. Predicting probable Alzheimer’s disease using linguistic deficits and biomarkers. BMC Bioinformatics. 2017;18(1):1–13.
König A, Linz N, Tröger J, Wolters M, Alexandersson J, Robert P. Fully automatic speech-based analysis of the semantic verbal fluency task. Dement Geriatr Cogn Disord. 2018;45(3–4):198–209.
Jiang Z, Seyedi S, Haque RU, Pongos AL, Vickers KL, Manzanares CM, et al. Automated analysis of facial emotions in subjects with cognitive impairment. PLoS ONE. 2022;17(1): e0262527.
Gattupalli S, Ebert D, Papakostas M, Makedon F, Athitsos V. Cognilearn: a deep learning-based interface for cognitive behavior assessment. In: Proceedings of the 22nd International Conference on Intelligent User Interfaces; 2017. p. 577–87.
Fei Z. Investigation of computer vision techniques for automatic detection of mild cognitive impairment in the elderly. University of Strathclyde; 2020. PhD Thesis.
Öztürk Ö, Akgül CB, Erçil A, Şahiner M, Şahiner T. Automatic analysis of hands clapping in severe Alzheimer patient via computer vision techniques. Alzheimers Dement. 2011;7(4).
Ahmed OB, Benois-Pineau J, Allard M, Catheline G, Amar CB, Initiative ADN, et al. Recognition of Alzheimer’s disease and Mild Cognitive Impairment with multimodal image-derived biomarkers and Multiple Kernel Learning. Neurocomputing. 2017;220:98–110.
Meng X, Yu H, Tham MP, Gait phase detection in able-bodied subjects and dementia patients. In: 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE;2013. p. 4907–10.
Alsubai S, Alqahtani A, Sha M, Abbas S, Almadhor A, Peter V, et al. Smart home-based complex interwoven activities for cognitive health assessment. Journal of Sensors. 2022;2022.
Khan MA, Khan A, Alhaisoni M, Alqahtani A, Alsubai S, Alharbi M, et al. Multimodal brain tumor detection and classification using deep saliency map and improved dragonfly optimization algorithm. International Journal of Imaging Systems and Technology. 2022.
Alam MAU, Roy N, Holmes S, Gangopadhyay A, Galik E. Autocognisys: Iot assisted context-aware automatic cognitive health assessment. In: MobiQuitous 2020-17th EAI International Conference on Mobile and Ubiquitous Systems: Computing, Networking and Services; 2020. p. 184-95.
Chen R, Jankovic F, Marinsek N, Foschini L, Kourtis L, Signorini A, et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. In: Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining; 2019. p. 2145-55.
Bringas S, Salomón S, Duque R, Montaña JL, Lage C. A convolutional neural network-based method for human movement patterns classification in Alzheimer’s disease. In: Multidisciplinary Digital Publishing Institute Proceedings. vol. 31; 2019. p. 72.
Delmastro F, Di Martino F, Dolciotti C. Cognitive training and stress detection in mci frail older people through wearable sensors and machine learning. IEEE Access. 2020;8:65573–90.
Alharbi EA, Jones JM, Alomainy A. Non-invasive solutions to identify distinctions between healthy and mild cognitive impairments participants. IEEE J Transl Eng Health Med. 2022.
Xie H, Wang Y, Tao S, Huang S, Zhang C, Lv Z. Wearable sensor-based daily life walking assessment of gait for distinguishing individuals with amnestic mild cognitive impairment. Front Aging Neurosci. 2019;11:285.
Shahzad A, Dadlani A, Lee H, Kim K. Automated Prescreening of mild cognitive impairment using shank-mounted inertial sensors based gait biomarkers. IEEE Access. 2022;10:15835–44.
Mancioppi G, Fiorini L, Critelli ML, Rovini E, Sportiello MT, Cavallo F, Evaluation of MCI motor performances during a cognitive dual task exercise. In 2019 IEEE 23rd International Symposium on Consumer Technologies (ISCT). IEEE; 2019. p. 247–50.
Gao H, Zhou L, Kim JY, Li Y, Huang W. The behavior guidance and abnormality detection for A-MCI patients under wireless sensor network. ACM Trans Sens Netw. 2021.
Alberdi A, Weakley A, Schmitter-Edgecombe M, Cook DJ, Aztiria A, Basarab A, et al. Smart home-based prediction of multidomain symptoms related to Alzheimer’s disease. IEEE J Biomed Health Inform. 2018;22(6):1720–31.
Sokullu R, Akkaş MA, Demir E. IoT supported smart home for the elderly. Internet of Things. 2020;11: 100239.
Lussier M, Lavoie M, Giroux S, Consel C, Guay M, Macoir J, et al. Early detection of mild cognitive impairment with in-home monitoring sensor technologies using functional measures: a systematic review. IEEE J Biomed Health Inform. 2018;23(2):838–47.
Zhao W, Pillai JA, Leverenz JB, Luo X. Technology-facilitated detection of mild cognitive impairment: a review. In: 2018 IEEE International Conference on Electro/Information Technology (EIT). IEEE; 2018. p. 0284–9.
Lauraitis A, Maskeliūnas R, Damaševičius R, Połap D, Woźniak M. A smartphone application for automated decision support in cognitive task based evaluation of central nervous system motor disorders. IEEE J Biomed Health Inform. 2019;23(5):1865–76.
Li VO, Lam JC, Han Y, Cheung LY, Downey J, Kaistha T, et al. Designing a protocol adopting an artificial intelligence (AI)-driven approach for early diagnosis of late-onset Alzheimer’s disease. J Mol Neurosci. 2021;71(7):1329–37.
Kalafatis C, Modarres MH, Apostolou P, Marefat H, Khanbagi M, Karimi H, et al. Validity and cultural generalisability of a 5-minute AI-based, computerised cognitive assessment in Mild Cognitive Impairment and Alzheimer’s Dementia. Front Psych. 2021;12:1155.
Choi W, Lee S. Ground kayak paddling exercise improves postural balance, muscle performance, and cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. Medical Science Monitor: International Medical Journal of Experimental And Clinical Research. 2018;24:3909.
Cavedoni S, Chirico A, Pedroli E, Cipresso P, Riva G. Digital biomarkers for the early detection of mild cognitive impairment: artificial intelligence meets virtual reality. Front Hum Neurosci. 2020;14:245.
Tsai CF, Chen CC, Wu EHK, Chung CR, Huang CY, Tsai PY, et al. A machine-learning-based assessment method for early-stage neurocognitive impairment by an immersive virtual supermarket. IEEE Trans Neural Syst Rehabil Eng. 2021;29:2124–32.
Caggianese G, Chirico A, De Pietro G, Gallo L, Giordano A, Predazzi M, et al. Towards a virtual reality cognitive training system for mild cognitive impairment and Alzheimer’s disease patients. In: 2018 32nd International Conference on Advanced Information Networking and Applications Workshops (WAINA). IEEE; 2018. p. 663–7.
Riaz W, Khan ZY, Jawaid A, Shahid S. Virtual reality (VR)-based environmental enrichment in older adults with mild cognitive impairment (MCI) and mild Dementia. Brain Sci. 2021;11(8):1103.
Naz S, Ashraf A, Zaib A. Transfer learning using freeze features for Alzheimer neurological disorder detection using ADNI dataset. Multimedia Syst. 2022;28(1):85–94.
Basaia S, Agosta F, Wagner L, Canu E, Magnani G, Santangelo R, et al. Automated classification of Alzheimer’s disease and mild cognitive impairment using a single MRI and deep neural networks. NeuroImage: Clinical. 2019;21:101645.
Lin CY, Chen CH, Tom SE, Kuo SH. Cerebellar volume is associated with cognitive decline in mild cognitive impairment: results from ADNI. The Cerebellum. 2020;19(2):217–25.
Lu L, Wang H, Elbeleidy S, Nie F. Predicting cognitive declines using longitudinally enriched representations for imaging biomarkers. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition; 2020. p. 4827-36.
Almubark I, Alsegehy S, Jiang X, Chang LC. Early Detection of Mild Cognitive Impairment using Neuropsychological Data and Machine Learning Techniques. In: 2020 IEEE Conference on Big Data and Analytics (ICBDA). IEEE; 2020. p. 32-7.
Patient R, Ghali F, Kolivand H, Hurst W, John N. Application of virtual reality and electrodermal activity for the detection of cognitive impairments. In: 2021 14th International Conference on Developments in eSystems Engineering (DeSE). IEEE; 2021. p. 156–61.
Bloch L, Friedrich CM. Data analysis with Shapley values for automatic subject selection in Alzheimer’s disease data sets using interpretable machine learning. Alzheimers Res Ther. 2021;13(1):1–30.
Maserejian N, Bian S, Wang W, Jaeger J, Syrjanen JA, Aakre J, et al. Practical algorithms for amyloid \(\beta\) probability in subjective or mild cognitive impairment. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2019;11:710–20.
Liu Y, Yue L, Xiao S, Yang W, Shen D, Liu M. Assessing clinical progression from subjective cognitive decline to mild cognitive impairment with incomplete multi-modal neuroimages. Med Image Anal. 2022;75: 102266.
Zhou X, Qiu S, Joshi PS, Xue C, Killiany RJ, Mian AZ, et al. Enhancing magnetic resonance imaging-driven Alzheimer’s disease classification performance using generative adversarial learning. Alzheimers Res Ther. 2021;13(1):1–11.
Liu M, Zhang J, Adeli E, Shen D. Joint classification and regression via deep multi-task multi-channel learning for Alzheimer’s disease diagnosis. IEEE Trans Biomed Eng. 2018;66(5):1195–206.
Basheer S, Bhatia S, Sakri SB. Computational modeling of dementia prediction using deep neural network: analysis on OASIS dataset. IEEE Access. 2021;9:42449–62.
Tomassini S, Falcionelli N, Sernani P, Müller H, Dragoni AF, An end-to-end 3D ConvLSTM-based framework for early diagnosis of Alzheimer’s disease from full-resolution whole-brain sMRI scans. In: 2021 IEEE 34th International Symposium on Computer-Based Medical Systems (CBMS). IEEE; 2021. p. 74–8.
Salehi AW, Baglat P, Sharma BB, Gupta G, Upadhya A. A CNN model: earlier diagnosis and classification of Alzheimer disease using MRI. In: 2020 International Conference on Smart Electronics and Communication (ICOSEC). IEEE; 2020. p. 156–61.
Castellano G, Esposito A, Mirizio M, Montanaro G, Vessio G, Detection of Dementia through 3D convolutional neural networks based on amyloid PET. In: 2021 IEEE Symposium Series on Computational Intelligence (SSCI). IEEE; 2021. p. 1–6.
Liu L, Hu X, Zhu L, Fu CW, Qin J, Heng PA. \(\psi\)-net: stacking densely convolutional LSTMS for sub-cortical brain structure segmentation. IEEE Trans Med Imaging. 2020;39(9):2806–17.
Yamanakkanavar N, Choi JY, Lee B. MRI segmentation and classification of human brain using deep learning for diagnosis of Alzheimer’s disease: a survey. Sensors. 2020;20(11):3243.
Roy AG, Conjeti S, Navab N, Wachinger C, Initiative ADN, et al. QuickNAT: A fully convolutional network for quick and accurate segmentation of neuroanatomy. Neuroimage. 2019;186:713–27.
Dolz J, Desrosiers C, Ayed IB. 3D fully convolutional networks for subcortical segmentation in MRI: a large-scale study. Neuroimage. 2018;170:456–70.
Mehta R, Christinck T, Nair T, Bussy A, Premasiri S, Costantino M, et al. Propagating uncertainty across cascaded medical imaging tasks for improved deep learning inference. IEEE Trans Med Imaging. 2021;41(2):360–73.
Ataloglou D, Dimou A, Zarpalas D, Daras P. Fast and precise hippocampus segmentation through deep convolutional neural network ensembles and transfer learning. Neuroinformatics. 2019;17(4):563–82.
Liang L, Zhou P, Lu W, Guo X, Ye C, Lv H, et al. An anatomical knowledge-based MRI deep learning pipeline for white matter hyperintensity quantification associated with cognitive impairment. Comput Med Imaging Graph. 2021;89: 101873.
Azkune G, Almeida A. A scalable hybrid activity recognition approach for intelligent environments. IEEE Access. 2018;6:41745–59.
Almeida A, Azkune G. Predicting human behaviour with recurrent neural networks. Appl Sci. 2018;8(2):305.
Tax N. Human activity prediction in smart home environments with LSTM neural networks. In: 2018 14th International Conference on Intelligent Environments (IE). IEEE; 2018. p. 40–7.
Arifoglu D, Bouchachia A. Abnormal behaviour detection for dementia sufferers via transfer learning and recursive auto-encoders. In: 2019 IEEE International Conference on Pervasive Computing and Communications Workshops (PerCom Workshops). IEEE; 2019. p. 529–34.
Zolfaghari S, Khodabandehloo E, Riboni D. TraMiner: Vision-based analysis of locomotion traces for cognitive assessment in smart-homes. Cogn Comput. 2021:1–22.
Khodabandehloo E, Riboni D. Collaborative trajectory mining in smart-homes to support early diagnosis of cognitive decline. IEEE Trans Emerg Top Comput. 2020;9(3):1194–205.
Khodabandehloo E, Riboni D, Alimohammadi A. HealthXAI: collaborative and explainable AI for supporting early diagnosis of cognitive decline. Futur Gener Comput Syst. 2021;116:168–89.
Yahaya SW, Lotfi A, Mahmud M. A consensus novelty detection ensemble approach for anomaly detection in activities of daily living. Appl Soft Comput. 2019;83: 105613.
Dehzangi O, Sahu V. IMU-based robust human activity recognition using feature analysis, extraction, and reduction. In: 2018 24th International Conference on Pattern Recognition (ICPR). IEEE; 2018. p. 1402-7.
Cho H, Yoon SM. Divide and conquer-based 1D CNN human activity recognition using test data sharpening. Sensors. 2018;18(4):1055.
Wan S, Qi L, Xu X, Tong C, Gu Z. Deep learning models for real-time human activity recognition with smartphones. Mob Netw Appl. 2020;25(2):743–55.
Micucci D, Mobilio M, Napoletano P. Unimib shar: a dataset for human activity recognition using acceleration data from smartphones. Appl Sci. 2017;7(10):1101.
Ferrari A, Micucci D, Mobilio M, Napoletano P. Trends in human activity recognition using smartphones. J Reliab Intell Environ. 2021;7(3):189–213.
Saha SS, Rahman S, Rasna MJ, Islam AM, Ahad MAR. DU-MD: An open-source human action dataset for ubiquitous wearable sensors. In: 2018 Joint 7th International Conference on Informatics, Electronics & Vision (ICIEV) and 2018 2nd International Conference on Imaging, Vision & Pattern Recognition (icIVPR). IEEE; 2018. p. 567-72.
Saini R, Kumar P, Kaur B, Roy PP, Dogra DP, Santosh K. Kinect sensor-based interaction monitoring system using the BLSTM neural network in healthcare. Int J Mach Learn Cybern. 2019;10(9):2529–40.
Ye Z, Hu S, Li J, Xie X, Geng M, Yu J, et al. Development of the cuhk elderly speech recognition system for neurocognitive disorder detection using the Dementia bank corpus. In: ICASSP 2021-2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP). IEEE; 2021. p. 6433–7.
Pan Y, Nallanthighal VS, Blackburn D, Christensen H, Härmä A. Multi-task estimation of age and cognitive decline from speech. In: ICASSP 2021-2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP). IEEE; 2021. p. 7258-62.
Fu Z, Haider F, Luz S, Predicting mini-mental status examination scores through paralinguistic acoustic features of spontaneous speech. In: 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). IEEE; 2020. p. 5548–52.
Lotfidereshgi R, Gournay P. Cognitive coding of speech. In: ICASSP 2022-2022 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP). IEEE; 2022. p. 7772-6.
Pan Y, Mirheidari B, Harris JM, Thompson JC, Jones M, Snowden JS, et al. Using the Outputs of Different Automatic Speech Recognition Paradigms for Acoustic-and BERT-Based Alzheimer’s Dementia Detection Through Spontaneous Speech. In: Interspeech. 2021. p. 3810–4.
Vaghari D, Bruna R, Hughes LE, Nesbitt D, Tibon R, Rowe JB, et al. A multi-site, multi-participant magnetoencephalography resting-state dataset to study Dementia: the BioFIND dataset. NeuroImage. 2022:119344.
Hughes LE, Henson RN, Pereda E, Bruña R, López-Sanz D, Quinn AJ, et al. Biomagnetic biomarkers for dementia: a pilot multicentre study with a recommended methodological framework for magnetoencephalography. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2019;11:450–62.
Alsaedi A, Abdel-Qader I, Mohammad N, Fong AC, Extended cox proportional hazard model to analyze and predict conversion from mild cognitive impairment to Alzheimer’s disease. In 2018 IEEE 8th Annual Computing and Communication Workshop and Conference (CCWC). IEEE; 2018. p. 131–6.
Hannah S, Deepa A, Chooralil VS, BrillySangeetha S, Yuvaraj N, Arshath Raja R, et al. Blockchain-based deep learning to process IoT data acquisition in cognitive data. Biomed Res Int. 2022;2022.
Zhornitsky S, Chaudhary S, Le TM, Chen Y, Zhang S, Potvin S, et al. Cognitive dysfunction and cerebral volumetric deficits in individuals with Alzheimer’s disease, alcohol use disorder, and dual diagnosis. Psychiatry Res Neuroimaging. 2021;317: 111380.
Al-Khafajiy M, Otoum S, Baker T, Asim M, Maamar Z, Aloqaily M, et al. Intelligent control and security of fog resources in healthcare systems via a cognitive fog model. ACM Trans Internet Technol (TOIT). 2021;21(3):1–23.
El Zarif O, Haraty RA. Toward information preservation in healthcare systems. In: Innovation in Health Informatics. Elsevier; 2020. p. 163-85.
Alazab M, RM SP, Parimala M, Reddy P, Gadekallu TR, Pham QV. Federated learning for cybersecurity: concepts, challenges and future directions. IEEE Trans Ind Inf. 2021.
Gadekallu TR, Manoj M, Kumar N, Hakak S, Bhattacharya S, et al. Blockchain-based attack detection on machine learning algorithms for IoT-based e-Health applications. IEEE Internet of Things Magazine. 2021;4(3):30–3.
Bzdok D, Krzywinski M, Altman N. Machine learning: a primer. Nat Methods. 2017;14(12):1119.
Nguyen DC, Pham QV, Pathirana PN, Ding M, Seneviratne A, Lin Z, et al. Federated learning for smart healthcare: a survey. ACM Comput Surv (CSUR). 2022;55(3):1–37.
Gadekallu TR, Pham QV, Huynh-The T, Bhattacharya S, Maddikunta PKR, Liyanage M. Federated learning for big data: a survey on opportunities, applications, and future directions. arXiv:2110.04160 [Preprint]. 2021. http://arxiv.org/abs/2110.04160.
Rieke N, Hancox J, Li W, Milletari F, Roth HR, Albarqouni S, et al. The future of digital health with federated learning. NPJ Digit Med. 2020;3(1):1–7.
Mingming Y, Bolun Z, Zhijian L, Yingli W, Lanshu Z. Effectiveness of computer-based training on post-stroke cognitive rehabilitation: a systematic review and meta-analysis. Neuropsychol Rehabil. 2022;32(3):481–97.
Ortelli P, Ferrazzoli D, Versace V, Cian V, Zarucchi M, Gusmeroli A, et al. Optimization of cognitive assessment in Parkinsonisms by applying artificial intelligence to a comprehensive screening test. npj Parkinson’s Disease. 2022;8(1):1-9.
Mazzei D, Chiarello F, Fantoni G. Analyzing social robotics research with natural language processing techniques. Cogn Comput. 2021;13(2):308–21.
Maddikunta PKR, Pham QV, Prabadevi B, Deepa N, Dev K, Gadekallu TR, et al. Industry 5.0: a survey on enabling technologies and potential applications. J Ind Inf Integr. 2022;26:100257.
Reddy GT, Reddy MPK, Lakshmanna K, Kaluri R, Rajput DS, Srivastava G, et al. Analysis of dimensionality reduction techniques on big data. IEEE Access. 2020;8:54776–88.
Shani R, Tal S, Derakshan N, Cohen N, Enock PM, McNally RJ, et al. Personalized cognitive training: protocol for individual-level meta-analysis implementing machine learning methods. J Psychiatr Res. 2021;138:342–8.
Ramu SP, Boopalan P, Pham QV, Maddikunta PKR, Huynh-The T, Alazab M, et al. Federated learning enabled digital twins for smart cities: Concepts, recent advances, and future directions. Sustain Cities Soc. 2022;79:103663.
Rahman MA, Brown DJ, Shopland N, Burton A, Mahmud M. Explainable multimodal machine learning for engagement analysis by continuous performance test. In: Universal Access in Human-Computer Interaction. User and Context Diversity: 16th International Conference, UAHCI 2022, Held as Part of the 24th HCI International Conference, HCII 2022, Virtual Event, June 26–July 1, 2022, Proceedings, Part II. Springer; 2022. p. 386-99.
Javed AR, Ahmed W, Pandya S, Maddikunta PKR, Alazab M, Gadekallu TR. A survey of explainable artificial intelligence for smart cities. Electronics. 2023;12(4).
Srivastava G, Jhaveri RH, Bhattacharya S, Pandya S, Maddikunta PKR, Yenduri G, et al. XAI for Cybersecurity: state of the art, challenges, open issues and future directions. arXiv:2206.03585 [Preprint]. 2022. http://arxiv.org/abs/2206.03585.
Wang S, Qureshi MA, Miralles-Pechuaán L, Huynh-The T, Gadekallu TR, Liyanage M. Explainable AI for B5G/6G: technical aspects, use cases, and research challenges. arXiv:2112.04698 [Preprint]. 2021. http://arxiv.org/abs/2112.04698.
Funding
MM is supported by the European Commission through projects funded under the Erasmus+ programme with agreement numbers 2020-1-UK01-KA201-079167 (AI-TOP) and 618615-EPP-1-2020-1-UKEPPKA2-CBHEJP (DiversAsia).
Author information
Authors and Affiliations
Contributions
This work was carried out in close collaboration between all co-authors. ARJ, TRG, and MM first defined the research theme and contributed an early design of the study. AS, HM, and PKRM further implemented and refined the study. ARJ, AS, TRG, MR, HM, and PKRM wrote the paper. MR, MM, ML, and AH edited the paper. All authors have contributed to, seen and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
In this study, no human or animal participants were involved, hence ethical approval was not needed.
Informed Consent
As this study did not involve any human participant, informed consent was not needed.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Javed, A.R., Saadia, A., Mughal, H. et al. Artificial Intelligence for Cognitive Health Assessment: State-of-the-Art, Open Challenges and Future Directions. Cogn Comput 15, 1767–1812 (2023). https://doi.org/10.1007/s12559-023-10153-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12559-023-10153-4