Abstract
Integrins are cell receptors involved in several metabolic pathways often associated with cell proliferation. Some of these integrins are downregulated during human physical development, but when these integrins are overexpressed in adult humans, they can be associated with several diseases, such as cancer. Molecules that specifically bind to these integrins are useful for cancer detection, diagnosis, and treatment. This review focuses on the structures of integrin-peptidic ligand complexes to dissect how the binding occurs and the molecular basis of the specificity and affinity of these peptidic ligands. Understanding these interactions at the molecular level is fundamental to be able to design new peptides that are more specific and more sensitive to a particular integrin. The integrin complexes covered in this review are α5β1, αIIbβ3, αvβ3, αvβ6, and αvβ8, because the molecular structures of the complex have been experimentally determined and their presence on tumor cancer cells are associated with a poor prognosis, making them targets for cancer detection and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Integrins are α and β heterodimeric transmembrane receptors expressed on various cell types and tissues throughout the body, playing important roles in cell adhesion, migration, signaling, motility, proliferation, polarity, differentiation, and survival. Despite the similarities in amino acid sequences and structures, the 24 integrins (built with 18α- and 8β-subunits) have different ligand-binding specificities.
Changes in the expression pattern of certain integrins contribute to cancer development by promoting tumor cell survival, metastasis, invasion, and extravasation. This review will focus on integrins involved in cancer development, having the three-dimensional structure determined in complexes with a peptidic ligand (see also Table 1). Understanding the molecular basis of ligand affinity and specificity for these integrins is helpful in designing molecules for tumor detection and treatment with high affinity and specificity (Urquiza et al. 2020; Takada et al. 2007; Desgrosellier and Cheresh 2010; Barczyk et al. 2010; Slack et al. 2022).
Integrin α5β1
Drug-resistant ovarian cancers have significantly higher α5β1-integrin expression than partially sensitive or sensitive ovarian cancers (Hu and Gao 2012). Moreover, the α5-integrin is overexpressed in 9% of ovarian cancer patients, and they have a lower survival rate (Sawada et al. 2008). Additionally, integrin α5 plays a crucial role in mediating cancer cell-fibroblast interactions during peritoneal dissemination of diffuse-type gastric carcinoma (DGC), which is associated with poor prognosis. Monoclonal antibodies (mab) recognizing integrin α5β1 inhibit the adhesion of DGC cells to cancer-associated fibroblasts, reducing diffusive infiltration, frequent peritoneal dissemination, and massive fibrosis (Miyamoto et al. 2022). Moreover, high levels of integrin α5β1 and galectin-1 in stromal cells are associated with no-response to cisplatin-based neoadjuvant chemotherapy in squamous cervical cancer patients (Zhu et al. 2017).
In contrast, integrin α5β1 expression is frequently lost in colorectal cancer cells, which is associated with proliferation and tumorigenicity involving HER-2 signaling. In fact, integrin α5β1 re-expression in colon cancer cells abrogates their tumorigenicity by increasing lysosomal targeting of HER-2 (Kuwada et al. 2005). Furthermore, integrin α5β1 binding to the secreted protein Tubulointerstitial nephritis antigen-like 1 (Tinagl1), which also involves αvβ1 and epidermal growth factor receptor (EGFR), results in suppressing triple-negative breast cancer progression and metastasis (Shen et al. 2019; Trerotola et al. 2013). There are several antagonists of integrin α5β1 in clinical trials, and the most successful ones are as follows:
-
1.
ATN-161 (Ac-PHSCN-NH2), an integrin α5β1 antagonist, inhibits tumor angiogenesis and metastasis in various tumor types. ATN-161 blocks integrin α5β1 interactions, strongly inhibiting nuclear factor-κB (NF-κB) activation and matrix metalloproteinase-2/9 expression (Sui et al. 2018)
-
2.
Cilengitide (Merck KGaA), a cyclic RGD pentapeptide that blocks the RGD integrin binding site, greatly hinders angiogenesis, starving the tumor, and in specific cases, improving patient treatment efficacy and outcome
-
3.
Volociximab, a high-affinity chimeric antibody against human α5β1-integrin, inhibits fibronectin binding and endothelial cell survival and proliferation. In a xenografted ovarian cancer model, volociximab significantly reduced tumor burden
The crystal structure of integrin α5β1 in complex with RGD-peptide at a resolution of 1.78 Å, downloaded from the Protein Data Bank (PDB: 4WK0)(Xia and Springer 2014), shows several water molecules between α5 and β1-subunits and magnesium and calcium ions (Fig. 1). These water molecules not only help to bind the two chains together but also to bind the RGD-peptide to both integrin subunits. The Arg-residue of RGD-peptide makes hydrogen bonds with two water molecules connected to F-187, S-224, and D-227 residues from α5-subunit. The Gly-residue of the RGD-peptide interacts with three water molecules, S-227 from β1-subunit, also F-187 from α5-subunit. The Asp-residue of the RGD-peptide interacts with four water molecules, two magnesium ions and N-224, and E-229 from β1-subunit. Additionally, it makes contact with a water molecule and D-227 from α5-subunit and a magnesium ion. Asp from RGD-peptide binds to a water molecule and to the β1-subunit residues G-223, and N-224 directly plus S-132, and E-229 through a magnesium ion.
Integrin αIIbβ3
The integrin αIIbβ3 is the most abundant receptor on platelets, which binds to peptides and proteins containing the RGD sequence present on different adhesive proteins, including fibrinogen, VWF, fibronectin, and vitronectin. The integrin αIIbβ3 is also involved in cancer progression, tumor cell proliferation, metastasis, and promoting tumor cell adhesion and invasion. Additionally, αIIbβ3 facilitates interaction with tumor cells through binding with podoplanin, P-selectin glycoprotein ligand-1 (PSGL-1), A disintegrin and metalloproteinase domain-containing protein 9 (ADAM-9), and fibrinogen/αvβ3. This interaction probably creates a physical shield around cancer cells, protecting them from the immune system (Boukerche et al. 1989; Grossi et al. 1988; Honn et al. 1992; Timar et al. 1998; Nierodzik et al. 1992; Wagner et al. 1996; Mammadova-Bach et al. 2016; Tesfamariam 2016).
The crystal structure of integrin αIIBβ3 in complex with GRGDSP peptide has been obtained at a resolution of 3.00 Å (PDB: 3ZE1) (Zhu et al. 2013) (Fig. 2). The structure reveals three manganese ions bound to the β3 subunit in close proximity to the GRGDSP peptide binding site, with one of them bound to S-121, S-123, and D-119. The Arg and the Asp from GRGDSP peptide interact with the αIIB and the β3-subunit, respectively. The complex structure is stabilized by water molecules, and there is a salt bridge between R-216 from the β3-subunit and E-123 from the αIIB subunit near the contact site of the GRGDSP peptide.
The Arg from GRGDSP peptide interacts with the αIIB-subunit through bidentate hydrogen bonds with D-224 and a water molecule, as well as with F-160. It also has aromatic and hydrophobic interactions with Y-189, Y-190, F-231, and L-192. The first Gly of GRGDSP peptide interacts with RGD Y-190 from the αIIB-subunit, as well as A-218 from the β3-subunit. The Asp from GRGDSP peptide interacts with the β3-subunit through residues S-121, Y-122, S-123, N-215, and E-220 and a manganese ion. The residues SP from GRGDSP peptide interact with the β3-subunit through residues Y-122 and S-123, while Pro interacts with D-126, which is bound to another manganese ion (Zhu et al. 2013).
Integrin αvβ3
Abnormal integrin αvβ3 expression is linked to cancer progression, tumor initiation, sustained tumor growth, distant metastasis, drug resistance development, and maintenance of stemness in cancer cells. The αvβ3-integrin is overexpressed in melanoma, breast cancer, and ovarian cancer. Several integrin αvβ3 antagonists have been developed, including RGD-4 C (ACDCRGDCFCG), a double cysteine-bridged peptide containing RGD, a PEGylated bicyclic single-cysteine bridging peptide, KCRGDCFC based on RGD-4 C, LXW7, LXZ2, IsoDGR, the isoDGR-based cyclopeptide CisoDGRC, linear small peptides RWrNM and RWrNK (containing d-arginine that can pass through the blood-brain tumor barrier), ProAgio (interacting outside the RGD binding site), and the RGD cyclic peptide cilengitide, the most clinically studied αvβ3 inhibitor molecule (Hynes 2002; Stupp et al. 2014; Gu et al. 2023)
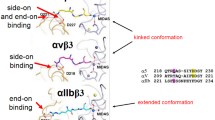
The αvβ3 ligand-binding pocket contains three bivalent metal ion binding sites. The metal ion-dependent adhesion site directly coordinates the side chain of acidic residue characteristics of all integrin ligands, whereas the two external sites can also bind Mn2+, Mg2+, and Ca2+. This integrin has three basic conformations: bending (low-affinity ligand binding), medium affinity, and extended (high-affinity ligand binding). Integrin conformational changes are associated to divalent cations, activating antibodies (e.g., CBRLFA1/2), ligand-mimicking peptides, or intracellular activators (Van Agthoven et al. 2014; Xiao et al. 2004; Valdramidou et al. 2008; Zhu et al. 2013; Takagi et al. 2002; Nishida et al. 2006; Shattil et al. 2010; Ye et al. 2012; Schürpf and Springer 2011).
The Arg-Gly-Asp (RGD) sequence is the smallest integrin-binding motif that has been used for the development of numerous peptides and small molecules targeting integrins, such as αvβ3. These range from the linear tripeptide RGD (Kd = 89 nM) and the heptapeptide GRGDSPK (Kd = 12.2 nM) to cyclic RGD peptides with improved binding characteristics (more stable, effective, and specific than linear RGD peptides). Additionally, allosteric inhibitors of αvβ3 have also been developed, such as C19-9, a non-RGD inhibitor of αvβ3 with good affinity and excellent drug-like characteristics (Pierschbacher and Ruoslahti 1984; Indrevoll et al. 2006; Meyer et al. 2006; Sheldrake and Patterson 2014; Auzzas et al. 2010; Shimaoka and Springer 2003; Kapp et al. 2017; Pang et al. 2021, 2023; Gu et al. 2023).
Targeting both αvβ3 and α5β1 integrins is more effective in cancer treatment than targeting only one of them. For example, knottins 2.5D and 2.5F have nanomolar affinity for binding to αvβ3 (2.5D) or both αvβ3 and α5β1 (2.5F). The difference between 2.5D and 2.5F is four residues located aside of the RGD motif. The 2.5F and 2.5D binding to αvβ3 depends critically on the RGD loop flexibility (Van Agthoven et al. 2019; Kapp et al. 2017; Coller and Shattil 2008; Mason 2015; Caswell et al. 2008; Christoforides et al. 2012; Reynolds et al. 2009; Moore et al. 2013). Moreover, the human mab intetumumab (also known as CNTO 95) recognizes all αv-integrin family member (Kd = 210 ± 133 pM for αvβ3 and Kd = 250 ± 104 pM for αvβ5) (Trikha et al. 2004) and has anti-angiogenic and anti-tumor properties, inhibiting cell adhesion, migration, proliferation, and invasion. LM609, a mouse anti-human mab against αvβ3-integrin, has displayed significant anti-angiogenic activity in preclinical trials (Kobayashi et al. 2017; Almokadem and Belani 2012; Mitra et al. 2011; O'Day et al. 2011; Huang et al. 2013)
Integrin αvβ6
The integrin αvβ6, which binds to vitronectin, tenascin-C, and fibronectin, plays a role in cell adhesion and migration. It is typically expressed at low levels or absent in healthy adult tissue epithelia but specifically upregulated during tissue repair, embryogenesis, and carcinogenesis. It also binds to latency-associated peptides (LAPs), resulting in the release of active TGF-β from the latent complex (Breuss et al. 1995; Busk et al. 1992; Dong et al. 2017; Larjava et al. 2011; Brzozowska and Deshmukh 2022; Urquiza et al. 2020).
High levels of αvβ6 expression in cancer patients with carcinomas of the skin, stomach, colon, breast, lung, oral mucosa, cervix, salivary gland, liver, ovary, and endometrium are usually associated with metastasis, tumor invasion, and a decrease in the median survival time of patients. High levels of αvβ6 expression combined with MMP-9, eIF4E, or Ets-1 are a prognostic indicator in patients with gastric cancer, colorectal cancer, and non-small cell lung cancer. Thus, integrin αvβ6 is a potential target for cancer treatment and diagnosis (Ahmed et al. 2002a, b; Berghoff et al. 2014; Koivisto et al. 2018; Lian et al. 2016; Niu et al. 2014; Niu and Li 2017; Peng et al. 2014; Wang and Hielscher 2017; Brzozowska and Deshmukh 2022). In fact, the peptide A20FMDV2 (NAVPNLRGDLQVLAQKVART), derived from the foot and mouth virus, exhibits high selectivity and affinity for the αvβ6 integrin, allowing αvβ6+ tumor detection using single-photon emission computed tomography (SPECT) and positron emission tomography (PET). This peptide, radiolabeled with 4-[18F] fluorobenzoic acid, is utilized in microPET for cancer imaging of αvβ6+ tumor cells (Brzozowska and Deshmukh 2022; Saleem et al. 2020; Hausner and Bold 2019).
The A20 peptide, incorporated into the DG loop of the HAdV-D10 fiber knob, enables the virus to selectively target and eradicate αvβ6+ tumor cells. Furthermore, the oncolytic adenovirus AdΔΔ modified with the A20FMDV2 selectively targets αvβ6 integrin and specifically eliminates αvβ6+ tumor cells. In fact, this virus is highly selective for αvβ6+ pancreatic cancer cells, which is particularly promising in treating metastasis of the incurable αvβ6+ pancreatic ductal adenocarcinoma (Bates et al. 2022; Man et al. 2018).
The αvβ6 integrin ligands have been used for designing chimeric antigen receptor (CAR) cells, to eradicate αvβ6+ cancer cells. The A20-2G and A20-4G CAR-constructs have been designed for targeting cholangiocarcinoma, a deadly form of bile duct cancer. These CARs contain peptide A20 fused with a second-generation CD28/CD3ζ signaling domain or with a fourth-generation CD28/4-1BB/CD27/CD3ζ signaling domain. Another CAR T cell construct, co-expressing CXCR2, the cognate IL-8 receptor, shows superior anti-tumor activity against αvβ6+ ovarian or pancreatic tumor xenografts. Furthermore, a CAR construct containing A20 peptide fused to CD28 + CD3 endodomain co-expressing an IL-4-responsive fusion gene (4αβ) is very potent in vivo in mice harboring αvβ6+ ovarian, breast, and pancreatic tumor xenografts. Conversely, a CAR construct has been built with a high-affinity 12-mer peptide (Bpep) specific for αvβ6 integrin and a human IgG4 hinge-Fc extracellular domain fused to the cytoplasmic tail of CD3-zeta. Primary human cytotoxic T lymphocytes expressing this CAR selectively eliminate αvβ6+ ovarian tumor cells. (Phanthaphol et al. 2021; Whilding et al. 2017, 2019; Uusi-Kerttula et al. 2018; Pameijer et al. 2007).
Integrin αvβ8
The αvβ8 integrin binds specifically to the RGD peptide, present in vitronectin, collagen IV, and the main ligand latent TGFβ1/3. Initially, Itgb8 gene expression is detectable in most organs except for adipose tissue and blood. The αvβ8 integrin-mediated regulation of TGFβ signaling is crucial for the normal functions of immune cells and plays a role in the initiation and progression of various cancers. The reduced αvβ8 integrin expression correlates with higher-grade tumors in squamous cell carcinomas, whereas upregulation of β8 integrin expression in pancreatic cancer cells leads to more malignant disease and enhanced resistance to chemotherapy. Moreover, β8 integrin expression promotes perivascular growth of glioblastoma (GBM) cells and their invasion along blood vessels. GBM cell lines expressing low levels of integrin β8 are less invasive, while those expressing high levels of integrin β8 increase tumor growth and perivascular invasion. The αvβ8 integrin plays a pro-tumorigenic role in GBM stem cells, promoting tumor recurrence following radiation and chemotherapy; and there is a correlation between αvβ8 integrin expression and the severity of metastatic lesions in metastatic brain tumors (McCarty 2020; Reyes et al. 2013; Tchaicha et al. 2011; Lathia et al. 2015; Malric et al. 2019; Schittenhelm et al. 2013).
Structural insights of peptide ligand molecular interaction with integrins αvβ3, αvβ6, and αvβ8
Numerous crystal structures of integrins αvβ3, αvβ6, and αvβ8 in complex with RGD-peptides have been obtained by X-ray diffraction (Xiong et al. 2002; Dong et al. 2014; Wang et al. 2019). Here, we analyze the crystal structures found in the protein data bank of integrin αvβ3 in complex with cyclic RGDF-peptide at a resolution of 3.20 Å (PDB: 1L5G) (Xiong et al. 2002), integrin αvβ6 in complex with GRGDLGRLKK-peptide at a resolution of 2.50 Å (PDB: 4UM9) (Dong et al. 2014), and integrin αvβ8 in complex with pro TGF-β1 peptide GRRGDLATIH at a resolution of 2.77 Å (PDB: 6OM2) (Wang et al. 2019). The Arg residue from the RGD motif, present in the peptides binding to these integrin, interacts with the α subunit, while the Asp from this motif interacts with the β subunit (Fig. 3).
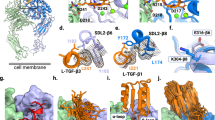
(a) Structure of the extracellular segment of integrin αvβ3 in complex with cyclic RGD-peptide (αv-subunit in green, β3-subunit in red, and RGD peptide in blue) with its interaction map of the RGD-peptide (C) with the α5-subunit (A) and β-3 subunit (B). (b) Structure of integrin αvβ6 in complex with latency-associated peptide (αv-subunit in green, β6-subunit in red, and latency-associated peptide in blue) with its interaction map of the RGD-peptide (E) with the α5-subunit (A) and β-3 subunit (B). (c) Structure of integrin αvβ8 in complex with pro TGF-β1 peptide (αv-subunit in green, β8-subunit in red, and pro TGF-β1 peptide in blue) with its interaction map of the RGD-peptide (F) with the α5-subunit (C) and β-3 subunit (D)
The structures of the αv-subunit in the integrins αvβ3, αvβ6, and αvβ8 are very similar, with few changes in the position of main chain atoms. The peptide binding site on these integrins is also very similar. In fact, the R-residue of the RGD-motif contained in these peptides interacts with αv-subunit residues Y-178 and D-218 of these three integrins, D-150 with integrins αvβ3 and αvβ8, Q-180 with integrins αvβ6 and αvβ8, and F-177 with integrin αvβ8. The Arg from cyclic RGDF-peptide interacts with W-179 of the αv-subunit. However, changes in the position of several side chains of the αv-subunit, probably caused by interactions with the β-subunit, lead to changes in the αv-residues that interact with the R-residue of the RGD motif.
Additionally, the G-residue of the RGD-motif interacts with residues from the β-subunit in these three structures: R-216 and A-218 from the β3-subunit, T-221 and I-219 from the β6-subunit, and I-218 and T-210 from the β8-subunit. Meanwhile, the D-residue of the RGD-motif is buried and interacts with a pocket formed by residues S-121, N-215, R-216, E-220, and two manganese ions in the β3-subunit; by residues S-125, A-126, P-179, I-183, A-217, N-218, I-219, E-223, and one calcium and one magnesium ion in the β6-subunit; and with residues S-114, A-115, S-116, G-206, N-207, I-208, E-212, and one calcium and magnesium ion in the β8-subunit. The F residue of the RGDF cyclic peptide forms an aromatic interaction with Y-122 from the β3-subunit, which is not observed in the β6- and β8-subunits.
The first Arg of peptide GRRGDLAT interacts with Y-172, and Asp from RGD interacts with D-216 from β8-subunits. In addition, a hydrophobic pocket in the β6-subunit formed by an intramolecular disulfide bond between the C-180 and C-187 residues (also present in β3 and β8), and neighboring residues interact with L-214 and L-217 from the GRGDLGRLKK peptide. The molecular characteristics of this pocket vary among the three integrins, and according to experimental evidence, it is involved in the specificity of binding of these integrins. Furthermore, the structure indicates that the binding site for the C-terminus of the GRGDLGRLKK and GRRGDLATIH peptides is located in this pocket on the αvβ6 and αvβ8 integrins, respectively.
Also, the first Gly-residue of the GRRGDLAT peptide interacts with D-150 and Y-178 from the αv-subunit, while the first Arg of this peptide forms a hydrogen bond with Y-172 from the β8-subunit. The Leu-residue of the GRRGDLAT peptide interacts with A-115 from the β8-subunit, and the Thr-residue of the same peptide interacts with Ser-116 and His-118 from the β8-subunit (Wang et al. 2019).
A salt bridge is formed between the αv and β3 subunits through the interaction of an αv chain Asp and a β3 chain Lys, near the contact site of the RGD-peptide as reported by Xiong et al. (2002). Similarly, Dong et al. (2014) observed a salt bridge between the αv-subunit D-148 and β6-subunit K-170.
Characteristics of the surface area buried on the integrin after peptide complex formation
In the five complexes analyzed here, the buried surface area of the peptides on the integrins ranges from 204.1 to 556.7 Å2, with mostly side chain atoms involved (between 77.2% and 96.1%), which is crucial for binding specificity to different integrins. Additionally, the integrin surface area buried is predominantly hydrophobic (between 60.7% and 73.4%), despite the main interaction being salt bridges involving the R and D residues in the peptide ligands (see also Table 2). Therefore, when designing an integrin-specific peptide or small molecule ligand, it is necessary to consider not only the RGD residues but also the surrounding residues, as they are responsible for the peptide’s specificity. Although the RGD residues are the main integrin binders, the surrounding peptide residues can cause steric hindrance, which may modify the specificity of peptide binding.
References
Ahmed N, Pansino F et al (2002a) Association between alphavbeta6 integrin expression, elevated p42/44 kDa MAPK, and plasminogen-dependent matrix degradation in ovarian cancer. J Cell Biochem 84(4):675–686. https://doi.org/10.1002/jcb.10080
Ahmed N, Riley C et al (2002b) Alpha(v)beta(6) integrin-a marker for the malignant potential of epithelial ovarian cancer. J Histochem Cytochem 50(10):1371–1380. https://doi.org/10.1177/002215540205001010
Almokadem S, Belani CP (2012) Volociximab in cancer. Expert Opin Biol Ther 12(2):251–257. https://doi.org/10.1517/14712598.2012.646985
Auzzas L, Zanardi F et al (2010) Targeting alphavbeta3 integrin: design and applications of mono- and multifunctional RGD-based peptides and semipeptides. Curr Med Chem 17(13):1255–1299. https://doi.org/10.2174/092986710790936301
Barczyk M, Carracedo S, Gullberg D (2010) Integrins. Cell Tissue Res 339(1):269–280. https://doi.org/10.1007/s00441-009-0834-6
Bates EA, Davies JA et al (2022) Development of a low-seroprevalence, αvβ6 integrin-selective virotherapy based on human adenovirus type 10. Mol Ther Oncolytics 25:43–56. https://doi.org/10.1016/j.omto.2022.03.007
Berghoff AS, Kovanda AK et al (2014) αvβ3, αvβ5 and αvβ6 integrins in brain metastases of lung cancer. Clin Exp Metastasis 31(7):841–851. https://doi.org/10.1007/s10585-014-9675-0
Boukerche H, Berthier-Vergnes O et al (1989) Platelet-melanoma cell interaction is mediated by the glycoprotein IIb-IIIa complex. Blood 74(2):658–663. https://doi.org/10.1182/blood.V74.2.658.658
Breuss JM, Gallo J et al (1995) Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci 108(Pt 6):2241–2251. https://doi.org/10.1242/jcs.108.6.224
Brzozowska E, Deshmukh S (2022) Integrin alpha v beta 6 (αvβ6) and its implications in cancer treatment. Int J Mol Sci 23(20):12346. https://doi.org/10.3390/ijms232012346
Busk M, Pytela R, Sheppard D (1992) Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem 267(9):5790–5796. https://doi.org/10.1016/S0021-9258(18)42622-1
Caswell PT, Chan M et al (2008) Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 183(1):143–155. https://doi.org/10.1083/jcb.200804140
Christoforides C, Rainero E et al (2012) PKD controls αvβ3 integrin recycling and tumor cell invasive migration through its substrate rabaptin-5. Dev Cell 23(3):560–572. https://doi.org/10.1016/j.devcel.2012.08.008
Coller BS, Shattil SJ (2008) The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood 112(8):3011–3025. https://doi.org/10.1182/blood-2008-06-077891
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10(1):9–22. https://doi.org/10.1038/nrc2748
Dong X, Hudson NE et al (2014) Structural determinants of integrin β-subunit specificity for latent TGF-β. Nat Struct Mol Biol 21(12):1091–1096. https://doi.org/10.1038/nsmb.2905
Dong X, Zhao B et al (2017) Force interacts with macromolecular structure in activation of TGF-β. Nature 542(7639):55–59. https://doi.org/10.1038/nature21035
Grossi IM, Hatfield JS et al (1988) Role of tumor cell glycoproteins immunologically related to glycoproteins Ib and IIb/IIIa in tumor cell-platelet and tumor cell-matrix interactions. FASEB J 2(8):2385–2395. https://doi.org/10.1096/fasebj.2.8.2452113
Gu Y, Dong B et al (2023) The challenges and opportunities of αvβ3-based therapeutics in cancer: from bench to clinical trials. Pharmacol Res 189:106694. https://doi.org/10.1016/j.phrs.2023.106694
Hausner SH, Bold RJ (2019) Preclinical development and first-in-human imaging of the integrin αvβ6 with [18F]αvβ6-binding peptide in metastatic carcinoma. Clin Cancer Res 25(4):1206–1215. https://doi.org/10.1158/1078-0432.CCR-18-2665
Honn KV, Chen YQ et al (1992) Alpha IIb beta 3 integrin expression and function in subpopulations of murine tumors. Exp Cell Res 201(1):23–32. https://doi.org/10.1016/0014-4827(92)90344-8
Hu Z, Gao S (2012) Elevated levels of Lewis y and integrin α5β1 correlate with chemotherapeutic drug resistance in epithelial ovarian carcinoma. Int J Mol Sci 13(12):15588–15600. https://doi.org/10.3390/ijms131215588
Huang J, Zhang J et al (2013) VCAM1 expression correlated with tumorigenesis and poor prognosis in high grade serous ovarian cancer. Am J Transl Res 5(3):336–346
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110(6):673–687. https://doi.org/10.1016/s0092-8674(02)00971-6
Indrevoll B, Kindberg GM et al (2006) NC-100717: a versatile RGD peptide scaffold for angiogenesis imaging. Bioorganic Med Chem Lett 16(24):6190–6193. https://doi.org/10.1016/j.bmcl.2006.09.033
Kapp TG, Rechenmacher F et al (2017) A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep 7:39805. https://doi.org/10.1038/srep39805
Kobayashi M, Sawada K, Kimura T (2017) Potential of integrin inhibitors for treating ovarian cancer: a literature review. Cancers 9(7):83. https://doi.org/10.3390/cancers9070083
Koivisto L, Bi J, Häkkinen L, Larjava H (2018) Integrin αvβ6: structure, function and role in health and disease. Int J Biochem Cell Biol 99:186–196. https://doi.org/10.1016/j.biocel.2018.04.013
Kuwada SK, Kuang J, Li X (2005) Integrin alpha5/beta1 expression mediates HER-2 down-regulation in colon cancer cells. J Biol Chem 280(19):19027–19035. https://doi.org/10.1074/jbc.M410540200
Larjava H, Koivisto L et al (2011) Epithelial integrins with special reference to oral epithelia. J Dent Res 90(12):1367–1376. https://doi.org/10.1177/0022034511402207
Lathia JD, Mack SC et al (2015) Cancer stem cells in glioblastoma. Genes Dev 29(12):1203–1217. https://doi.org/10.1101/gad.261982.115
Lian PL, Liu Z et al (2016) Integrin αvβ6 and matrix metalloproteinase 9 correlate with survival in gastric cancer. World J Gastroenterol 22(14):3852–3859. https://doi.org/10.3748/wjg.v22.i14.3852
Malric L, Monferran S et al (2019) Inhibiting integrin β8 to differentiate and radiosensitize glioblastoma-initiating cells. Mol Cancer Res 17(2):384–397. https://doi.org/10.1158/1541-7786.MCR-18-0386
Mammadova-Bach E, Zigrino P et al (2016) Platelet integrin α6β1 controls lung metastasis through direct binding to cancer cell-derived ADAM9. JCI Insight 1(14):e88245. https://doi.org/10.1172/jci.insight.88245
Man YKS, Davies JA et al (2018) The novel oncolytic adenoviral mutant Ad5-3Δ-A20T retargeted to αvβ6 integrins efficiently eliminates pancreatic cancer cells. Mol Cancer Ther 17(2):575–587. https://doi.org/10.1158/1535-7163.MCT-17-0671
Mason WP (2015) End of the road: confounding results of the CORE trial terminate the arduous journey of cilengitide for glioblastoma. Neuro-oncology 17(5):634–635. https://doi.org/10.1093/neuonc/nov018
McCarty JH (2020) αvβ8 integrin adhesion and signaling pathways in development, physiology and disease. J Cell Sci 133(12):jcs239434. https://doi.org/10.1242/jcs.239434
Meyer A, Auernheimer J et al (2006) Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des 12(22):2723–2747. https://doi.org/10.2174/138161206777947740
Mitra AK, Sawada K et al (2011) Ligand-independent activation of c-Met by fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene 30(13):1566–1576. https://doi.org/10.1038/onc.2010.532
Miyamoto S, Nagano et al (2022) Integrin α5 mediates cancer cell-fibroblast adhesion and peritoneal dissemination of diffuse-type gastric carcinoma. Cancer Lett 526:335–345. https://doi.org/10.1016/j.canlet.2021.11.008
Moore SJ, Hayden Gephart MG et al (2013) Engineered knottin peptide enables noninvasive optical imaging of intracranial medulloblastoma. Proc Natl Acad Sci U.S.A 110(36):14598–14603. https://doi.org/10.1073/pnas.1311333110
Nierodzik ML, Kajumo F, Karpatkin S (1992) Effect of thrombin treatment of tumor cells on adhesion of tumor cells to platelets in vitro and tumor metastasis in vivo. Cancer Res 52(12):3267–3272
Nishida N, Xie C, Shimaoka M et al (2006) Activation of leukocyte beta2 integrins by conversion from bent to extended conformations. Immunity 25(4):583–594. https://doi.org/10.1016/j.immuni.2006.07.016
Niu J, Li Z (2017) The roles of integrin αvβ6 in cancer. Cancer Lett 403:128–137. https://doi.org/10.1016/j.canlet.2017.06.012
Niu Z, Wang J et al (2014) Protein expression of eIF4E and integrin αvβ6 in colon cancer can predict clinical significance, reveal their correlation and imply possible mechanism of interaction. Cell Biosci 4:23. https://doi.org/10.1186/2045-3701-4-23
O'Day S, Pavlick A et al (2011) A randomised, phase II study of intetumumab, an anti-αv-integrin mAb, alone and with dacarbazine in stage IV melanoma. Br J Cancer 105(3):346–352. https://doi.org/10.1038/bjc.2011.183
Pameijer CR, Navanjo A et al (2007) Conversion of a tumor-binding peptide identified by phage display to a functional chimeric T cell antigen receptor. Cancer Gene Ther 14(1):91–97. https://doi.org/10.1038/sj.cgt.7700993
Pang X, Sun X et al (2023) Discovery of C19-9 as a novel non-RGD inhibitor of αvβ3 to overcome enzalutamide resistance in castration-resistant prostate cancer. Signal Transduct Target Ther 8(1):60. https://doi.org/10.1038/s41392-022-01236-z
Pang X, Zhang J et al (2021) SPP1 promotes enzalutamide resistance and epithelial-mesenchymal-transition activation in castration-resistant prostate cancer via PI3K/AKT and ERK1/2 pathways. Oxid Med Cell Longev 2021:5806602. https://doi.org/10.1155/2021/5806602
Peng C, Gao H et al (2014) Integrin αvβ6 and transcriptional factor Ets-1 act as prognostic indicators in colorectal cancer. Cell Biosci 4(1):53. https://doi.org/10.1186/2045-3701-4-53
Phanthaphol N, Somboonpatarakun C et al (2021) Chimeric antigen receptor T cells targeting integrin αvβ6 expressed on cholangiocarcinoma cells. Front Oncol 11:657868. https://doi.org/10.3389/fonc.2021.657868
Pierschbacher MD, Ruoslahti E (1984) Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309(5963):30–33. https://doi.org/10.1038/309030a0
Reyes SB, Narayanan AS et al (2013) αvβ8 integrin interacts with RhoGDI1 to regulate Rac1 and Cdc42 activation and drive glioblastoma cell invasion. Mol Biol Cell 24(4):474–482. https://doi.org/10.1091/mbc.E12-07-0521
Reynolds AR, Hart IR et al (2009) Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med 15(4):392–400. https://doi.org/10.1038/nm.194
Saleem A, Helo Y et al (2020) Integrin αvβ6 positron emission tomography imaging in lung cancer patients treated with pulmonary radiation therapy. Int J Radiat Oncol Biol Phys 107(2):370–376. https://doi.org/10.1016/j.ijrobp.2020.02.014
Sawada K, Mitra AK et al (2008) Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res 68(7):2329–2339. https://doi.org/10.1158/0008-5472.CAN-07-5167
Schittenhelm J, Klein A et al (2013) Comparing the expression of integrins αvβ3, αvβ5, αvβ6, αvβ8, fibronectin and fibrinogen in human brain metastases and their corresponding primary tumors. Int J Clin Exp Pathol 6(12):2719–2732
Schürpf T, Springer TA (2011) Regulation of integrin affinity on cell surfaces. The EMBO J 30(23):4712–4727. https://doi.org/10.1038/emboj.2011.333
Shattil SJ, Kim C, Ginsberg MH (2010) The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol 11(4):288–300. https://doi.org/10.1038/nrm2871
Sheldrake HM, Patterson LH (2014) Strategies to inhibit tumor associated integrin receptors: rationale for dual and multi-antagonists. J Med Chem 57(15):6301–6315. https://doi.org/10.1021/jm5000547
Shen M, Jiang YZ et al (2019) Tinagl1 suppresses triple-negative breast cancer progression and metastasis by simultaneously inhibiting integrin/FAK and EGFR signaling. Cancer cell 35(1):64–80.e7. https://doi.org/10.1016/j.ccell.2018.11.016
Shimaoka M, Springer TA (2003) Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov 2(9):703–716. https://doi.org/10.1038/nrd1174
Slack RJ, Macdonald SJF et al (2022) Emerging therapeutic opportunities for integrin inhibitors. Nat Rev Drug Discov 21(1):60–78. https://doi.org/10.1038/s41573-021-00284-4
Stupp R, Hegi ME, CENTRIC study team et al (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. The Lancet. Oncology 15(10):1100–1108. https://doi.org/10.1016/S1470-2045(14)70379-1
Sui A, Zhong Y et al (2018) ATN-161 as an integrin α5β1 antagonist depresses ocular neovascularization by promoting new vascular endothelial cell apoptosis. Med Sci Monit 24:5860–5873. https://doi.org/10.12659/MSM.907446
Takada Y, Ye X, Simon S (2007) The integrins. Genome Biol 8(5):215. https://doi.org/10.1186/gb-2007-8-5-215
Takagi J, Petre BM et al (2002) Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110(5). https://doi.org/10.1016/s0092-8674(02)00935-2
Tchaicha JH, Reyes SB et al (2011) Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by β8 integrin. Cancer Res 71(20):6371–6381. https://doi.org/10.1158/0008-5472.CAN-11-0991
Tesfamariam B (2016) Involvement of platelets in tumor cell metastasis. Pharmacol Ther 157:112–119. https://doi.org/10.1016/j.pharmthera.2015.11.005
Timar J, Trikha M et al (1998) Expression and function of the high affinity alphaIIbbeta3 integrin in murine melanoma cells. Clin Exp Metastasis 16(5):437–445. https://doi.org/10.1023/a:1006533508560
Trerotola M, Jernigan DL et al (2013) Trop-2 promotes prostate cancer metastasis by modulating β(1) integrin functions. Cancer Res 73(10):3155–3167. https://doi.org/10.1158/0008-5472.CAN-12-3266
Trikha M, Zhou Z, Nemeth JA, Chen Q, Sharp C, Emmell E, Giles-Komar J, Nakada MT (2004) CNTO 95, a fully human monoclonal antibody that inhibits alphav integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer J Int Du Cancer 110(3):326–335. https://doi.org/10.1002/ijc.20116
Urquiza M, Guevara V, Diaz-Sana E, Mora F (2020) The role of αvβ6 integrin binding molecules in the diagnosis and treatment of cancer. Curr Org Chem 24(21):2393–2411. https://doi.org/10.2174/1385272824999200528124936
Uusi-Kerttula H, Davies JA et al (2018) Ad5NULL-A20: a tropism-modified, αvβ6 integrin-selective oncolytic adenovirus for epithelial ovarian cancer therapies. Clin Cancer Res 24(17):4215–4224. https://doi.org/10.1158/1078-0432.CCR-18-1089
Valdramidou D, Humphries MJ, Mould AP (2008) Distinct roles of beta1 metal ion-dependent adhesion site (MIDAS), adjacent to MIDAS (ADMIDAS), and ligand-associated metal-binding site (LIMBS) cation-binding sites in ligand recognition by integrin alpha2beta1. J Biol Chem 283(47):32704–32714. https://doi.org/10.1074/jbc.M802066200
Van Agthoven JF, Shams H et al (2019) Structural basis of the differential binding of engineered knottins to integrins αVβ3 and α5β1. Structure (London, England : 1993) 27(9):1443–1451.e6. https://doi.org/10.1016/j.str.2019.06.011
Van Agthoven JF, Xiong JP et al (2014) Structural basis for pure antagonism of integrin αVβ3 by a high-affinity form of fibronectin. Nat Struct Mol Biol 21(4):383–388. https://doi.org/10.1038/nsmb.2797
Wagner CL, Mascelli MA et al (1996) Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood 88(3):907–914. https://doi.org/10.1182/blood.V88.3.907.907
Wang J, Su Y et al (2019) General structural features that regulate integrin affinity revealed by atypical αVβ8. Nat Commun 10(1):5481. https://doi.org/10.1038/s41467-019-13248-5
Wang JP, Hielscher A (2017) Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. J Cancer 8(4):674–682. https://doi.org/10.7150/jca.16901
Whilding LM, Halim L et al (2019) CAR T-cells targeting the integrin αvβ6 and co-expressing the chemokine receptor CXCR2 demonstrate enhanced homing and efficacy against several solid malignancies. Cancers 11(5):674. https://doi.org/10.3390/cancers11050674
Whilding LM, Parente-Pereira AC et al (2017) Targeting of aberrant αvβ6 integrin expression in solid tumors using chimeric antigen receptor-engineered T cells. Mol Ther 25(1):259–273. https://doi.org/10.1016/j.ymthe.2016.10.012
Xia W, Springer TA (2014) Metal ion and ligand binding of integrin α5β1. Proc Natl Acad Sci U.S.A 111(50):17863–17868. https://doi.org/10.1073/pnas.1420645111
Xiao T, Takagi J et al (2004) Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature 432(7013):59–67. https://doi.org/10.1038/nature02976
Xiong JP, Stehle T et al (2002) Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science (New York, N.Y.) 296(5565):151–155. https://doi.org/10.1126/science.1069040
Ye F, Kim C, Ginsberg MH (2012) Reconstruction of integrin activation. Blood 119(1):26–33. https://doi.org/10.1182/blood-2011-04-292128
Zhu H, Chen A et al (2017) Predictive role of galectin-1 and integrin α5β1 in cisplatin-based neoadjuvant chemotherapy of bulky squamous cervical cancer. Biosci Rep 37(5):BSR20170958. https://doi.org/10.1042/BSR20170958
Zhu J, Zhu J, Springer TA (2013) Complete integrin headpiece opening in eight steps. J Cell Biol 201(7):1053–1068. https://doi.org/10.1083/jcb.201212037
Acknowledgements
We would like to express our deep appreciation and gratitude to Universidad Nacional de Colombia for its generous support to our research group through the projects “Fortalecimiento de la alianza con la Pontificia Universidad Católica De Valparaíso para crear propuestas de uso de péptidos en la detección y tratamiento de enfermedades generadas por patógenos, particularmente SARS-CoV-2. (Hermes code: 52844)”. Without their financial assistance, this manuscript would not have been done.
Funding
Open Access funding provided by Colombia Consortium This project has been funded by the “Dirección de Investigación y extensión-Universidad Nacional de Colombia” through the project “Fortalecimiento de la alianza con la Pontificia Universidad Católica De Valparaíso para crear propuestas de uso de péptidos en la detección y tratamiento de enfermedades generadas por patógenos, particularmente SARS-CoV-2. (Hermes code: 52844)”.
Author information
Authors and Affiliations
Contributions
Mauricio Urquiza: he chose the topic for the review, contributed to the article’s design, and took the lead in writing it. Additionally, he assisted in the creation of tables, figures, and text and served as the coordinator for the paper’s development. Daniela Benavides: she assisted with the structural analysis of integrin complexes, contributed to the design of the figures, and participated in the writing of the article. Silvia Jiménez: she aided in the literature review of the role of integrins in cancer and contributed to the writing of the article.
Corresponding author
Ethics declarations
Ethical approval
Does not apply.
Consent to participate
Does not apply.
Consent for publication
Does not apply.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Urquiza, M., Benavides-Rubio, D. & Jimenez-Camacho, S. Structural analysis of peptide binding to integrins for cancer detection and treatment. Biophys Rev 15, 699–708 (2023). https://doi.org/10.1007/s12551-023-01084-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-023-01084-3