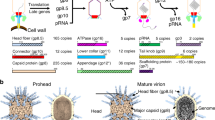
Abstract
Protein interactions in the assembly of the baseplate have been investigated. The baseplate of the phage T4 tail consists of a hub and six wedges which surround the former. Both reversible and irreversible interactions were found. Reversible association includes gp5 and gp27 (gp: gene product) which form a complex in a pH-dependent manner and gp18 polymerization, i.e. the tail sheath formation depends on the ionic strength. These reversible interactions were followed by irreversible or tight binding which pulls the whole association reaction to complete the assembly. The wedge assembly is strictly ordered which means that if one of the seven wedge proteins is missing, the assembly proceeds to that point and the remaining molecules stay non-associated. The strictly sequential assembly pathway is suggested to be materialized by successive conformational change upon binding, which can be shown by proteolytic probe.

Similar content being viewed by others
References
Abuladze NK, Gingery M, Tsai J, Eiserling FA (1994) Tail length determination in bacteriophage T4. Virology 199:301–310
Aksyuk AA, Leiman PG, Kurochkina LP, Shneider MM, Kostyuchenko VA, Mesyanzhinov VV, Rossmann MG (2009) The tail sheath structure of bacteriophage T4: a molecular machine for infecting bacteria. EMBO J 28:821–829
Arisaka F, Tschopp J, van Driel R, Engel J (1979) Reassembly of the bacteriophage T4 tail from the corebaseplate and the monomeric sheath protein P18: a cooperative association process. J Mol Biol 132:369–386
Epstein RH, Bolle A, Steinberg C, Kellenberger E, Boy de la Tour E, Chevalley R, Edgar R, Susman M, Denhart C, Lielausis I (1964) Physiological studies of conditional lethal mutants of bacteriophage T4D. Cold Spring Harb Symp Quant Biol 28:375–392
Ferguson PL, Coombs DH (2000) Pulse-chase analysis of the in vivo assembly of the bacteriophage T4 tail. J Mol Biol 297:99–117
Fokine A, Zhang Z, Kanamaru S, Bowman VD, Aksyuk AA, Arisaka F, Rao VB, Rossman MG (2013) The Molecular Architecture of the Bacteriophage T4 Neck. J Mol Biol. doi:10.1016/j.jmb.2013.02.012.
Fraenkel-Conrat H, Williams RC (1955) Reconstitution in vitro from coat protein and RNA. Proc Natl Acad Sci USA 41:690–698
Kanamaru S, Gassner NC, Ye N, Takeda S, Arisaka F (1999) The C-terminal fragment of the precursor tail lysozyme of bacteriophage T4 stays as a structural component of the baseplate after cleavage. J Bacteriol 181(9):2739–2744
Kanamaru S, Leiman PG, Kostyuchenko VA, Chipman PR, Mesyanzhinov VV, Arisaka F, Rossmann MG (2002) Structure of the cell-puncturing device of bacteriophage T4. Nature 415:553–557
Kanamaru S, Ishiwata Y, Suzuki T, Rossmann MG, Arisaka F (2005) Control of bacteriophage T4 tail lysozyme activity during the infection process. J Mol Biol 346:1013–1020
Kikuchi Y, King J (1975a) Genetic control of bacteriophage T4 baseplate morphogenesis I. Sequential assembly of the major precursor, in vivo and in vitro. J Mol Biol 99:645–672
Kikuchi Y, King J (1975b) Genetic control of bacteriophage T4 baseplate morphogenesis II. Mutants unable to form the central part of the baseplate. J Mol Biol 99:673–694
Kikuchi Y, King J (1975c) Genetic control of bacteriophage T4 baseplate morphogenesis III. J Mol Biol 99:695–716
Kostyuchenko VA, Leiman PG, Chipman PR, Kanamaru S, van Raaij MJ, Arisaka F, Mesyanzhinov VV, Rossmann MG (2003) Three-dimensional structure of bacteriophage T4 baseplate. Nat Struct Biol 10:688–693
Kostyuchenko VA, Chipman PR, Leiman PG, Arisaka F, Mesyanzhinov VV, Rossmann MG (2005) The tail structure of bacteriophage T4 and its mechanism of contraction. Nat Struct Mol Biol 12:810–813
Leiman P, Arisaka F, van Raaij MJ, Kostyuchenko VA, Aksyuk AA, Kanamaru S, Rossmann MG (2010) Morphogenesis of the T4 tail and tail fibers. Virol J 7:355–382
Nakagawa H, Arisaka F, Ishii S-I (1985) Isolation and characterization of the bacteriophage T4 tail-associated lysozyme. J Virol 54:460–466
Rao VB, Black LW (1988) Cloning, overexpression and purification of the terminase proteins gp16 and gp17 of bacteriophage T4: construction of defined in vitro DNA packaging system using purified terminase proteins. J Mol Biol 200:475–488
Rossmann MG, Arisaka F, Battisti AJ, Bowman VD, Chipman PR, Fokine A, Hafenstein S, Kanamaru S, Kostyuchenko VA, Mesyanzhinov VV, Shneider MM, Morais MC, Leiman PG, Palermo LM, Parrishd CR, Xiaoa C (2007) From structure of the complex to understanding of the biology. Acta Cryst D63:9–16
Sarkar SK, Takeda Y, Kanamaru S, Arisaka F (2006) Association and dissociation of the cell puncturing complex of bacteriophage T4 is controlled by both pH and temperature. Biochim Biophys Acta 1764:1487–1492
Showe MK, Isobe E, Onorato L (1976) Bacteriophage T4 Prehead proteinase. I. Purification and properties of a bacteriophage enzymewhich cleaves the capsid precursor protsins. J Mol Biol 107:35–54
Tschopp J, Arisaka F, van Driel R, Engel J (1979) Purification, characterization, and reassembly of the bacteriophage T4D tail sheath protein, P18. J Mol Biol 128:247–258
Yap ML, Mio K, Leiman PG, Kanamaru S, Arisaka F (2010a) The baseplate wedges of bacteriophage T4 spontaneously assemble into hubless baseplate-like structure in vitro. J Mol Biol 395:349–360
Yap ML, Mio K, Ali S, Minton A, Kanamaru S, Arisaka F (2010b) Sequential assembly of the wedge of the baseplate of phage T4 in the presence and absence of gp11 as monitored by analytical ultracentrifugation. Macromol Biosci 10:808–813
Zhao L, Takeda S, Leiman PG, Arisaka F (2000) Stoichiometry and inter-subunit interaction of the wedge initiation complex, gp10-gp11, of bacteriophage T4. Biochim Biophys Acta 1479(1–2):286–292
Zhao L, Kanamaru S, Chaidirek C, Arisaka F (2003) P15 and P3, the tail completion proteins of bacteriophage T4, both form hexameric rings. J Bacteriol 185:1693–1700
Acknowledgements
I first encountered Dr. Minton in the early summer of 1997 when I sent a question of mine to RASMB internet forum. I cannot recall what it was, but he kindly replied to my question and we started to exchange e-mails. I thought he was in the U.S.A., but actually he was at that time staying at Protein Research Institute of Osaka University. So, I invited him to give a seminar at our institute. The next year, I went to Melbourne to attend the Lorne Conference and its satellite meeting on Reversible Associations in Structural and Molecular Biology which was held at the university of Melbourne prior to the Lorne meeting. I saw him there again. Three years later in 2001, I had an opportunity to organize a small international conference in the framework of Keihanna International Conference on Molecular Biophysics and invited Allen as a guest speaker. In 2003, my proposal to invite him for three months as a visiting professor was accepted by our Graduate School. It was a very stimulating experience for me to talk daily to him. He came to Japan again in 2006 as a JSPS fellow for short term research communication, where he constructed a hand-made CG machine to attach to our light scattering machine. That was before commercial CG instrument, Calypso, was available. At the time of writing this article, Dr. Minton is visiting our laboratory for collaboration together with Dr. Damien Hall from the University of Tsukuba. I greatly appreciate his ever-lasting stimulating enthusiasm, discussion and encouragement.
Conflict of interest
There is no conflict of interest on this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: Protein–Protein and Protein–Ligand Interactions in Dilute and Crowded Solution Conditions. In Honor of Allen Minton’s 70th Birthday
Rights and permissions
About this article
Cite this article
Arisaka, F., Kanamaru, S. Protein interactions in the assembly of the tail of bacteriophage T4. Biophys Rev 5, 79–84 (2013). https://doi.org/10.1007/s12551-013-0114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-013-0114-2