Abstract
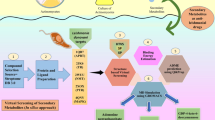
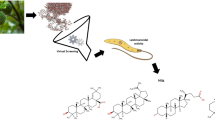
Leishmaniasis is a parasitic infection caused by unicellular protozoan organism belonging to the family Trypanosomatidae. Among various forms of the disease, visceral leishmaniasis is the most lethal and caused by Leishmania infantum or Leishmania donovani. The redox metabolism of parasite requires a key enzyme, trypanothione reductase which is a validated drug target. In the past decade, it was observed that these protozoan parasites had developed resistance against many of available drugs. Importantly in the case of visceral leishmaniasis drug resistance is very high in the Indian subcontinent, a major endemic region of Leishmania donovani infection. In search for new drugs, we aim to identify potential natural product inhibitors of trypanothione reductase which can be further developed as anti-leishmanial drug. We have performed in silico virtual screening of a natural product data set of 800 diverse chemical entities. Leishmania infantum trypanothione reductase crystal structure (PDB ID: 2JK6) was used in the virtual screening process, docking studies to identify potential lead compounds. The compounds were sorted based upon their binding energy and the top 50 ranked protein-inhibitor complexes were clustered using AuPosSOM to ligand foot print the interactions. We report a few alkaloids and sterols for the first time, which could be potential trypanothione reductase inhibitors. The footprinting of protein-inhibitor interactions into clusters has also provided clues on various possible orientations that inhibitors can attain at the active site of Trypanothione reductase. Moreover, biological significance of the interactions has also been discussed.
Similar content being viewed by others
References
Baiocco, P., Colotti, G., Franceschini, S., Andrea, I. 2009. Molecular basis of antimony treatment in leishmaniasis. J Med Chem 52, 2603–2612.
Bond, C.S., Zhang, Y., Berriman, M., Cunningham, M.L., Fairlamb, A.H., Hunter, A.N. 1999. Crystal structure of Trypanosoma cruzi trypanothione reductase in complex with trypanothione, and the structurebased discovery of new natural product inhibitors. Structure 7, 81–89.
Bouvier, G., Evrard-Todeschi, N., Girault, J.P., Bertho, G. 2010. Automatic clustering of docking poses in virtual screening process using self-organising map. Bioinformatics 26, 53–60.
Croft, S.L., Sundar, S., Fairlamb A.H. 2006. Drug resistance in leishmaniasis. Clin Microbiol Rev 19, 111–126.
Fairlamb, A.H., Blackburn, P., Ulrich, P., Chait, B.T., Cerami, A. 1985. Trypanothione: A novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science 227, 1485–1487.
Fairlamb, A.H., Cerami, A. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol 46, 695–729.
Holloway, G.A., Charman, W.N., Fairlamb, A.H., Brun, R., Kaiser, M., Kostewicz, E., Novello, P.M., Parisot, J.P., Richardson, J., Street, I.P., Watson, K.G., Baell J.B. 2009. Trypanothione reductase highthroughput screening campaign identifies novel classes of inhibitors with antiparasitic activity. Antimicrob Agents Chemother 53, 2824–2833.
Morris, G.M., Goodsell, D.S., Halliday, R.S., Huey, R., Hart, W.E., Belew, R.K., Olson, A.J. 1998. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19, 1639–1662.
Murray, H.W., Berman, J.D., Davies, C.R., Saravia, N.G. 2005. Advances in leishmaniasis. Lancet 366, 1561–1577.
Newman, D.J., Cragg, G.M. 2007. Natural poducts as sources of new drugs over the last 25 years. J Nat Prod 70, 461–477.
Orhan, I., Şener, B., Kaiser, M., Brun, R., Tasdemir, D. 2010. IInhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar Drugs 8, 47–58.
Perez-Pineiro, R., Burgos, A., Jones, D.C., Andrew, L.C., Rodriguez, H., Suarez, M., Fairlamb, A.H., Wishart, D.S. 2009. Development of a novel virtual screening cascade protocol to identify potential trypanothione reductase inhibitors. J Med Chem 52, 1670–1680.
Rivarola, H.W., Paglini-Oliva, P.A. 2002. Trypanosoma cruzi trypanothione reductase inhibitors: Phenothiazines and related compounds modify experimental Chagas’ disease evolution. Curr Drug Targets Cardiovasc Haematol Disord 2, 43–52.
Sanner, M.F. 1999. Python: A programming language for software integration and development. J Mol Graphics Mod 17, 57–61.
Shukla, A.K., Singh, B.K., Patra, S., Dubey, V.K. 2010. Rational approaches for drug designing against Leishmaniasis. Appl Biochem Biotechnol 160, 2208–2218.
Shukla, A.K., Patra, S., Dubey, V.K. 2011. Evaluation of selected antitumor agents as subversive substrate and potential inhibitor of trypanothione reductase: An alternative approach for chemotherapy of Leishmaniasis. Mol Cell Biochem, DOI: 10.1007/s11010-011-0762-0.
Tovar, J., Cunningham, M.I., Smith, A.C., Croft, S.I., Fairlamb, A.H. 1998. Down-regulation of Leishmania donovani trypanothione reductase by heterologous expression of a trans-dominant mutant homologue: Effect on parasite intracellular survival. Proc Natl Acad Sci USA 95, 5311–5316.
Venkatesan, S.K., Shukla, A.K., Dubey, V.K. 2010. Molecular docking studies of selected tricyclic and quinone derivatives on trypanothione reductase of Leishmania infantum. J Comput Chem 31, 2463–2472.
Vogt, A., Tamewitz, A., Skoko, J., Sikorski, R.P., Giuliano, K.A., Lazo, J.S. 2005. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem 280, 19078–19086.
Wallace, A.C., Laskowski, R.A., Thornton, J.M. 1995. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Prot Eng 8, 127–134.
Zhang, Y., Bond, C.S., Bailey, S., Cunningham, M.L., Fairlamb, A.H., Hunter, W.N. 1996. The crystal structure of trypanothione reductase from the human pathogen Trypanosoma cruzi at 2.3 Å resolution. Protein Sci 5, 52–61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatesan, S.K., Saudagar, P., Shukla, A.K. et al. Screening natural products database for identification of potential antileishmanial chemotherapeutic agents. Interdiscip Sci Comput Life Sci 3, 217–231 (2011). https://doi.org/10.1007/s12539-011-0101-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12539-011-0101-x