Abstract
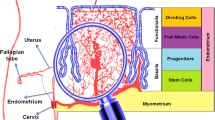
The human uterus is unique in that it possesses the tremendous regenerative capacity required for cyclical regeneration and remodeling throughout a woman’s reproductive life. Not only must the uterus rapidly enlarge to accommodate the developing fetus, the endometrium must also regenerate with each menstrual cycle. This plasticity of the reproductive system has recently been highlighted. My research group and collaborators showed that functional endometrial tissue could be regenerated from only a small number of singly dispersed human endometrial cells, transplanted beneath the kidney capsule of severely immunodeficient mice. This artificially generated endometrium resembles the natural endometrium, and contains human blood vessels that invade the mouse kidney parenchyma. Additionally, it mimics normal hormone-dependent changes including proliferation, differentiation, and tissue breakdown (menstruation). The regenerative capacity of endometrial cells makes them ideal candidates for tissue reconstitution, angiogenesis, and human–mouse chimeric vessel formation. The smooth muscle cells of the uterus (myometrium) share the plasticity of the endometrium. This is evidenced by their capacity for dramatic, repeatable, pregnancy-induced enlargement. Regeneration and remodeling in the female reproductive tract allude to the existence of endometrial and myometrial stem cell systems. We have recently isolated candidate populations of adult stem cells from both the human endometrium and myometrium. Characterization of these endometrial and myometrial cells, along with the study of the mechanisms controlling their regeneration, will improve the understanding of the physiology and pathophysiology of the female reproductive tract. Furthermore, myometrial and endometrial stem-like cells might also represent a novel source of biological material that could be used for the reconstruction of not only the human uterus but other organs as well.



Similar content being viewed by others
References
Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9.
Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–97.
Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update. 2007;13:87–101.
Ono M, Maruyama T, Yoshimura Y. Regeneration and adult stem cells in the human female reproductive tract. Stem Cells Cloning Adv Appl. 2008;1:23–9.
Maruyama T, Yoshimura Y. Molecular and cellular mechanisms for differentiation and regeneration of the uterine endometrium. Endocr J. 2008;55:795–810.
Padykula HA, Coles LG, Okulicz WC, Rapaport SI, McCracken JA, King NW Jr, et al. The basalis of the primate endometrium: a bifunctional germinal compartment. Biol Reprod. 1989;40:681–90.
Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–50.
Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46.
Strauss III JF, Lessey BA: The structure, function, and evaluation of the female reproductive tract. In: Strauss III JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology. Physiology, Pathophysiology, and Clinical Management, 5th ed. Amsterdam: Elsevier Saunders; 2004. p. 255–305.
Donnez J, Smoes P, Gillerot S, Casanas-Roux F, Nisolle M. Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 1998;13:1686–90.
Fujishita A, Hasuo A, Khan KN, Masuzaki H, Nakashima H, Ishimaru T. Immunohistochemical study of angiogenic factors in endometrium and endometriosis. Gynecol Obstet Invest. 1999;48(Suppl 1):36–44.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99.
Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79.
Zamah NM, Dodson MG, Stephens LC, Buttram VC Jr, Besch PK, Kaufman RH. Transplantation of normal and ectopic human endometrial tissue into athymic nude mice. Am J Obstet Gynecol. 1984;149:591–7.
Bergqvist A, Jeppsson S, Kullander S, Ljungberg O. Human uterine endometrium and endometriotic tissue transplanted into nude mice. Morphologic effects of various steroid hormones. Am J Pathol. 1985;121:337–41.
Zaino RJ, Satyaswaroop PG, Mortel R. Histologic response of normal human endometrium to steroid hormones in athymic mice. Hum Pathol. 1985;16:867–72.
Aoki D, Katsuki Y, Shimizu A, Kakinuma C, Nozawa S. Successful heterotransplantation of human endometrium in SCID mice. Obstet Gynecol. 1994;83:220–8.
Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99:2851–7.
Awwad JT, Sayegh RA, Tao XJ, Hassan T, Awwad ST, Isaacson K. The SCID mouse: an experimental model for endometriosis. Hum Reprod. 1999;14:3107–11.
Tabibzadeh S, Miller S, Dodson WC, Satyaswaroop PG. An experimental model for the endometriosis in athymic mice. Front Biosci. 1999;4:C4–9.
Grümmer R, Schwarzer F, Bainczyk K, Hess-Stumpp H, Regidor PA, Schindler AE, et al. Peritoneal endometriosis: validation of an in vivo model. Hum Reprod. 2001;16:1736–43.
Greenberg LH, Slayden OD. Human endometriotic xenografts in immunodeficient RAG-2/gamma(c)KO mice. Am J Obstet Gynecol. 2004;190:1788–95.
Fortin M, Lepine M, Merlen Y, Thibeault I, Rancourt C, Gosselin D, et al. Quantitative assessment of human endometriotic tissue maintenance and regression in a noninvasive mouse model of endometriosis. Mol Ther. 2004;9:540–7.
Matsuura-Sawada R, Murakami T, Ozawa Y, Nabeshima H, Akahira J, Sato Y, et al. Reproduction of menstrual changes in transplanted human endometrial tissue in immunodeficient mice. Hum Reprod. 2005;20:1477–84.
Masuda H, Maruyama T, Hiratsu E, Yamane J, Iwanami A, Nagashima T, et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/γ nullc immunodeficient mice. Proc Natl Acad Sci U S A. 2007;104:1925–30.
Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123:217–26.
Fujimoto J, Sakaguchi H, Hirose R, Wen H, Tamaya T. Angiogenesis in endometriosis and angiogenic factors. Gynecol Obstet Invest. 1999;48(Suppl 1):14–20.
Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann N Y Acad Sci. 2002;955:89–100. discussion 118, 396–406.
Nisolle M, Casanas-Roux F, Donnez J. Early-stage endometriosis: adhesion and growth of human menstrual endometrium in nude mice. Fertil Steril. 2000;74:306–12.
Bruner-Tran KL, Webster-Clair D, Osteen KG. Experimental endometriosis: the nude mouse as a xenographic host. Ann N Y Acad Sci. 2002;955:328–39.
Hull ML, Charnock-Jones DS, Chan CL, Bruner-Tran KL, Osteen KG, Tom BD, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88:2889–99.
Eggermont J, Donnez J, Casanas-Roux F, Scholtes H, Van Langendonckt A. Time course of pelvic endometriotic lesion revascularization in a nude mouse model. Fertil Steril. 2005;84:492–9.
St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202.
Bussolati B, Deambrosis I, Russo S, Deregibus MC, Camussi G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003;17:1159–61.
Dabrosin C, Gyorffy S, Margetts P, Ross C, Gauldie J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am J Pathol. 2002;161:909–18.
Nap AW, Griffioen AW, Dunselman GA, Bouma-Ter Steege JC, Thijssen VL, Evers JL, et al. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab. 2004;89:1089–95.
Seli E, Arici A. Endometriosis: interaction of immune and endocrine systems. Semin Reprod Med. 2003;21:135–44.
Yao L, Sgadari C, Furuke K, Bloom ET, Teruya-Feldstein J, Tosato G. Contribution of natural killer cells to inhibition of angiogenesis by interleukin-12. Blood. 1999;93:1612–21.
Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/γ nullc mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82.
Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–60.
Masuda H, Okano H, Maruyama T, Yoshimura Y, Okano H, Matsuzaki Y. In vivo imaging in humanized mice. Curr Top Microbiol Immunol. 2008;324:179–97.
Padykula HA. Regeneration in the primate uterus: the role of stem cells. Ann N Y Acad Sci. 1991;622:47–56.
Prianishnikov VA. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception. 1978;18:213–23.
Tanaka M, Kyo S, Kanaya T, Yatabe N, Nakamura M, Maida Y, et al. Evidence of the monoclonal composition of human endometrial epithelial glands and mosaic pattern of clonal distribution in luminal epithelium. Am J Pathol. 2003;163:295–301.
Brenner RM, Slayden OD, Rodgers WH, Critchley HO, Carroll R, Nie XJ, et al. Immunocytochemical assessment of mitotic activity with an antibody to phosphorylated histone H3 in the macaque and human endometrium. Hum Reprod. 2003;18:1185–93.
van Os R, Kamminga LM, de Haan G. Stem cell assays: something old, something new, something borrowed. Stem Cells. 2004;22:1181–90.
Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124–30.
Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11.
Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–806.
Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells. 2006;24:3–12.
Kato K, Yoshimoto M, Kato K, Adachi S, Yamayoshi A, Arima T, et al. Characterization of side-population cells in human normal endometrium. Hum Reprod. 2007;22:1214–23.
Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P, et al. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction. 2008;135:551–8.
Kim JY, Tavare S, Shibata D. Counting human somatic cell replications: methylation mirrors endometrial stem cell divisions. Proc Natl Acad Sci USA. 2005;102:17739–44.
Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292:81–5.
Ramsey EM. Anatomy of the human uterus. In: Chard T, Grudzinskas JG, editors. The uterus. Cambridge: Cambridge University Press; 1994. p. 18–40.
Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, et al. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod. 2006;74:839–49.
Ono M, Maruyama T, Masuda H, Kajitani T, Nagashima T, Arase T, et al. Side population in human uterine myometrium displays phenotypic and functional characteristics of myometrial stem cells. Proc Natl Acad Sci USA. 2007;104:18700–5.
Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–83.
Szotek PP, Chang HL, Zhang L, Preffer F, Dombkowski D, Donahoe PK, et al. Adult mouse myometrial label-retaining cells divide in response to gonadotropin stimulation. Stem Cells. 2007;25:1317–25.
Acknowledgments
I thank members of my research group, Yasunori Yoshimura, Hideyuki Okano, and Yumi Matsuzaki for their generous assistance and collaboration with this project. This study was supported, in part, by Grants-in-Aid from the Japan Society for the Promotion of Science (to T.M., Y.Y), by a National Grant-in-Aid for the Establishment of High-Tech Research Center in a Private University (to T.M.), and by a Grant-in-Aid from the 21st Century Centers of Excellence program of the Ministry of Education, Science, and Culture of Japan at Keio University.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Maruyama, T. Stem/progenitor cells and the regeneration potentials in the human uterus. Reprod Med Biol 9, 9–16 (2010). https://doi.org/10.1007/s12522-009-0032-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12522-009-0032-y