Abstract
Background
Chemotherapy and hematopoietic stem cell transplantation (HSCT) can damage the immune system, and may result in a loss of protection from infectious diseases. This study aimed to evaluate the impact of these treatments on the decrease in antibody titers of the measles, mumps, and rubella (MMR) vaccine and seroconversion post-revaccination of MMR.
Methods
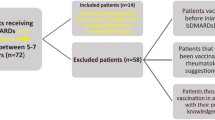
After completion of treatment for primary diseases, participants received an MMR revaccination. Antibody titers for MMR before revaccination were analyzed for all 110 children. After revaccination, 68 participants received a follow-up evaluation of antibody titer and adverse reaction.
Results
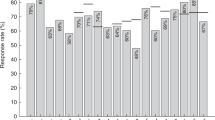
Multivariable analysis showed that therapeutic schedules were the only factor correlated with lack of antibody titers for measles after completing treatment (P = 0.008), while for mumps and rubella, no statistically significant difference was observed. Importantly, our study clearly demonstrated positive seroconversion rates for measles (97.5%), mumps (81.0%), and rubella (93.2%), with antibody levels rising across the board and peaking at around 6 months following revaccination. However, 6 months after revaccination, a downtrend of antibody titer levels was observed, which is comparatively earlier than the waning immunity observed in healthy children. Furthermore, we found MMR revaccination to be safe, with only a single adverse reaction (local pain at the injection site) reported.
Conclusions
MMR revaccination is immunogenic for the population. We suggest periodic monitoring of antibody titers, in addition to a booster vaccination, although the optimal timing of booster vaccination remains to be investigated further.


Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of nopho all2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32:606–15.
Mussolin L, Le Deley M-C, Carraro E, Damm-Welk C, Attarbaschi A, Williams D, et al. Prognostic factors in childhood anaplastic large cell lymphoma: long term results of the international alcl99 trial. Cancers. 2020;12:2747.
Baronciani D, Angelucci E, Potschger U, Gaziev J, Yesilipek A, Zecca M, et al. Hemopoietic stem cell transplantation in thalassemia: a report from the european society for blood and bone marrow transplantation hemoglobinopathy registry, 2000–2010. Bone Marrow Transplant. 2016;51:536–41.
Miyamoto S, Yanagimachi M, Umeda K, Iguchi A, Sasahara Y, Takada H, et al. Hematopoietic stem cell transplantation for severe combined immunodeficiency in japan: a nationwide retrospective analysis. Bone Marrow Transplant. 2020;55:443–543.
Perkins JL, Harris A, Pozos TC. Immune dysfunction after completion of childhood leukemia therapy. J Pediatr Hematol Oncol. 2017;39:1–5.
Friman V, Winqvist O, Blimark C, Langerbeins P, Chapel H, Dhalla F. Secondary immunodeficiency in lymphoproliferative malignancies. Hematol Oncol. 2016;34:121–32.
Goswami M, Prince G, Biancotto A, Moir S, Kardava L, Santich BH, et al. Impaired b cell immunity in acute myeloid leukemia patients after chemotherapy. J Transl Med. 2017;15:155.
Garonzi C, Balter R, Tridello G, Pegoraro A, Pegoraro M, Pacenti M, et al. The impact of chemotherapy after pediatric malignancy on humoral immunity to vaccine-preventable diseases. Mediterr J Hematol Infect Dis. 2020;12:e2020014.
Kuter BJ, Marshall GS, Fergie J, Schmidt E, Pawaskar M. Prevention of measles, mumps and rubella: 40 years of global experience with m-m-r-ii. Hum Vaccin Immunother. 2021;17:5372–83.
Measles vaccines. Who position paper—april 2017. Wkly Epidemiol Rec. 2017;92:205–27.
Mumps virus vaccines. Wkly Epidemiol Rec. 2007;82:51–60.
Progress in rubella and congenital rubella syndrome control and elimination—worldwide 2000–2016. Wkly Epidemiol Rec. 2017; 92:707–15.
Toret E, Yel SE, Suman M, Duzenli Kar Y, Ozdemir ZC, Dinleyici M, et al. Immunization status and re-immunization of childhood acute lymphoblastic leukemia survivors. Hum Vaccin Immunother. 2021;17:1132–5.
Kawamura K, Wada H, Nakasone H, Akahoshi Y, Kawamura S, Takeshita J, et al. Immunity and vaccination against measles, mumps, and rubella in adult allogeneic hematopoietic stem cell transplant recipients. Transplant Cell Ther. 2021;27:436.e1–e8.
Koochakzadeh L, Khosravi MH, Pourakbari B, Hosseinverdi S, Aghamohammadi A, Rezaei N. Assessment of immune response following immunization with dtp/td and mmr vaccines in children treated for acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2014;31:656–63.
Bochennek K, Allwinn R, Langer R, Becker M, Keppler OT, Klingebiel T, et al. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine. 2014;32:3357–61.
Esposito S, Prada E, Lelii M, Castellazzi L. Immunization of children with secondary immunodeficiency. Hum Vaccin Immunother. 2015;11:2564–70.
He HQ, Li Q, Yan R, Zhou Y, Tang XW, Deng X, et al. Antibody persistence following on different vaccination strategies of domestic measles, mumps and rubella combined attenuated live vaccine: a 3-year follow-up study. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51:336–40.
Olkinuora H, Kayhty H, Davidkin I, Roivainen M, Olander R-M, Kantele JM, et al. Immunity after (re)vaccination of paediatric patients following haematopoietic stem cell transplantation. Acta Paediatr. 2012;101:e373–7.
Patel SR, Ortin M, Cohen BJ, Borrow R, Irving D, Sheldon J, et al. Revaccination with measles, tetanus, poliovirus, haemophilus influenzae type b, meningococcus c, and pneumococcus vaccines in children after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:625–34.
Zignol M, Peracchi M, Tridello G, Pillon M, Fregonese F, D’elia R, et al. Assessment of humoral immunity to poliomyelitis, tetanus, hepatitis b, measles, rubella, and mumps in children after chemotherapy. Cancer. 2004;101:635–41.
Yildirim ZK, Buyukavci M. Assessment of humoral immunity to hepatitis b, measles, rubella, and mumps in children after chemotherapy. J Pediatr Hematol Oncol. 2018;40:E99–102.
Fouda AE, Kandil SM, Boujettif F, Salama YS, Fayea NY. Humoral immune response of childhood acute lymphoblastic leukemia survivors against the measles, mumps, and rubella vaccination. Hematology. 2018;23:590–5.
Viana SS, Araujo GS, Faro GBDA, Da Cruz-Silva LL, Araujo-Melo CA, Cipolotti R. Antibody responses to hepatitis b and measles-mumps-rubella vaccines in children who received chemotherapy for acute lymphoblastic leukemia. Rev Bras Hematol Hemoter. 2012;34:275–9.
Ek T, Josefson M, Abrahamsson J. Multivariate analysis of the relation between immune dysfunction and treatment intensity in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56:1078–87.
Inaba H, Hartford CM, Pei D, Posner MJ, Yang J, Hayden RT, et al. Longitudinal analysis of antibody response to immunization in paediatric survivors after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2012;156:109–17.
Shah GL, Shune L, Purtill D, Devlin S, Lauer E, Lubin M, et al. Robust vaccine responses in adult and pediatric cord blood transplantation recipients treated for hematologic malignancies. Biol Blood Marrow Transplant. 2015;21:2160–6.
King SM, Saunders EF, Petric M, Gold R. Response to measles, mumps and rubella vaccine in paediatric bone marrow transplant recipients. Bone Marrow Transplant. 1996;17:633–6.
Spoulou V, Giannaki M, Vounatsou M, Bakoula C, Grafakos S. Long-term immunity to measles, mumps and rubella after mmr vaccination among children with bone marrow transplants. Bone Marrow Transplant. 2004;33:1187–90.
Aytac S, Yalcin SS, Cetin M, Yetgin S, Gumruk F, Tuncer M, et al. Measles, mumps, and rubella antibody status and response to immunization in children after therapy for acute lymphoblastic leukemia. Pediatr Hematol Oncol. 2010;27:333–43.
Shaw PJ, Bleakley M, Burgess M. Safety of early immunization against measles/mumps/rubella after bone marrow transplantation. Blood. 2002;99:3486–586.
Gouveia-Alves F, Gouveia R, Ginani VC, Seber A, Kuramoto DA, Murad GFA, et al. Adherence and immune response to revaccination following hematopoietic stem cell transplantation at a pediatric onco-hematology reference center. Transpl Infect Dis. 2018;20:e12903.
Aurpibul L, Puthanakit T, Sirisanthana T, Sirisanthana V. Persistence of measles, mumps, and rubella protective antibodies 3 years after revaccination in hiv-infected children receiving antiretroviral therapy. Clin Infect Dis. 2010;50:1415–8.
Schenk J, Abrams S, Theeten H, Van Damme P, Beutels P, Hens N. Immunogenicity and persistence of trivalent measles, mumps, and rubella vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21:286–95.
Pandit A, Leblebjian H, Hammond SP, Laubach JP, Richardson PG, Baden LR, et al. Safety of live-attenuated measles-mumps-rubella and herpes zoster vaccination in multiple myeloma patients on maintenance lenalidomide or bortezomib after autologous hematopoietic cell transplantation. Bone Marrow Transpl. 2018;53:942–5.
Desjardins M, Mitre X, Sherman AC, Walsh SR, Cheng MP, Kanjilal S, et al. Safety of live-attenuated measles, mumps, and rubella vaccine administered within 2 years of hematopoietic cell transplant. Open Forum Infect Dis. 2021;8:504.
Aoki T, Kamimura T, Yoshida S, Mori Y, Kadowaki M, Kohno K, et al. Safety and seropositivity after live attenuated vaccine in adult patients receiving hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2019;25:1576–85.
Acknowledgements
The authors appreciate the assistance from the Department of Immunology, Shanghai Pudong New Area Center for Disease Control and Prevention, Shanghai, China and Department of Infectious Diseases, Shanghai Children's Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China. We would like to thank Editage (www.editage.cn) for English language editing.
Funding
None.
Author information
Authors and Affiliations
Contributions
WM and YQ contributed equally to this work. GYJ: conceptualization, writing–review and editing, supervision. CJ: conceptualization. ZH, ZF, CWJ and CQ: data curation. DPF and FY: investigation. WM: formal analysis, writing–original draft. YQ: formal analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approval
The study was approved by the Ethics Committee of Shanghai Children’s Medical Center (SCMCIRB‑K2017028) and registered at www.clinicaltrials.gov (NCT03373656). Informed consent to participate in the study have been obtained from parent or legal guardian of the participants (and themselves in the case of children over 8).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Yuan, Q., Deng, PF. et al. Measles, mumps, and rubella revaccination in children after completion of chemotherapy and hematopoietic stem cell transplantation: a single-center prospective efficacy and safety analysis. World J Pediatr 19, 1062–1070 (2023). https://doi.org/10.1007/s12519-023-00721-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-023-00721-x