Abstract
Introduction
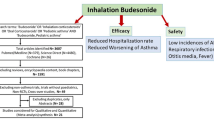
The efficacy of inhaled budesonide for managing moderate-to-severe acute exacerbations in children is not clear. Therefore, this study aimed to evaluate hospital admission rates, need for use of systemic corticosteroids, length of hospital stay and adverse events when inhaled budesonide is added to standard pediatric emergency department management of moderate-to-severe acute exacerbations of asthma.
Methods
A systematic search was conducted in PubMed, Scopus, CENTRAL (Cochrane Central Register of Controlled Trials) and Google scholar databases. Randomized controlled trials that evaluated the effect of nebulized budesonide in moderate-to-severe acute exacerbations of asthma in pediatric patients were included for this meta-analysis. Statistical analysis was done using STATA version 13.0.
Results
A total of 16 RCTs were included. Children receiving nebulized budesonide had 43% lower risk of being hospitalized (RR 0.57; 95% CI, 0.39; 0.85) and 66% lower risk of requiring systemic corticosteroids (RR 0.34; 95 % CI, 0.21; 0.55) compared with those receiving placebo. There were no differences in the length of hospital stay (Hedges’s g standardized mean difference − 1.53; 95% CI, − 3.64; 0.58) and risk of adverse events (RR 0.87, 95% CI; 0.65; 1.17) between the two groups. There was no evidence of publication bias for any of the outcomes considered.
Conclusion
The findings of this meta-analysis support the use of inhaled budesonide in reducing risk of hospitalization and the need for systemic corticosteroids among children with acute moderate-to-severe asthma exacerbation.





Similar content being viewed by others
References
Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–35.
Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–90.
NAE Asthma, PP. Third expert panel on the diagnosis and management of expert panel report 3: guidelines for the diagnosis and management of asthma. US: National Heart, Lung and Blood Institute; 2007.
Alangari AA. Corticosteroids in the treatment of acute asthma. Ann Thorac Med. 2014;9:187–92.
Rowe BH, Spooner C, Ducharme FM, Bretzlaff JA, Bota GW. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2000;(2):CD002178.
Pedersen S, Steffensen G, Ekman I, Tönnesson M, Borgå O. Pharmacokinetics of budesonide in children with asthma. Eur J Clin Pharmacol. 1987;31:579–82.
Papadopoulos NG, Arakawa H, Carlsen K-H, Custovic A, Gern J, Lemanske R, et al. International consensus on (ICON) pediatric asthma. Allergy. 2012;67:976–97.
Kearns N, Maijers I, Harper J, Beasley R, Weatherall M. Inhaled Corticosteroids in Acute Asthma: A Systemic Review and Meta-Analysis. J Allergy ClinImmunol Pract. 2019;8:605–17.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. England: Wiley; 2008.
Alangari AA, Malhis N, Mubasher M, Al-Ghamedi N, Al-Tannir M, Riaz M, et al. Budesonide nebulization added to systemic prednisolone in the treatment of acute asthma in children: a double-blind, randomized, controlled trial. Chest. 2014;145:772–8.
Chen A, Zeng G, Chen R, Zhan J, Sun L, Huang S, et al. Effects of nebulized high-dose budesonide on moderate-to-severe acute exacerbation of asthma in children: a randomized, double-blind, placebo-controlled study. Respirology. 2013;18(Suppl 3):47–52.
Chen A, Chen R, Zhan J, Huang S, Lin Y, Chen D, et al. The efficacy of nebulized budesonide in acute moderate to severe exacerbations of asthma in children. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:269–74.
Razi CH, Akelma AZ, Harmanci K, Kocak M, Kuras CY. The Addition of inhaled budesonide to standard therapy shortens the length of stay in hospital for asthmatic preschool children: a randomized, double-blind. Placebo-Controlled Trial Int Arch Allergy Immunol. 2015;166:297–303.
Razi CH, Cörüt N, Andıran N. Budesonide reduces hospital admission rates in preschool children with acute wheezing. Pediatr Pulmonol. 2017;52:720–8.
Sekerel BE, Sackesen C, Tuncer A, Adalioglu G. The effect of nebulized budesonide treatment in children with mild to moderate exacerbations of asthma. Acta Paediatr. 2005;94:1372–7.
Singhi S, Banerjee S, Nanjundaswamy H. Inhaled budesonide in acute asthma. J Paediatr Child Health. 1999;35:483–7.
Sung L, Osmond MH, Klassen TP. Randomized, controlled trial of inhaled budesonide as an adjunct to oral prednisone in acute asthma. Acad Emerg Med. 1998;5:209–13.
Upham BD, Mollen CJ, Scarfone RJ, Seiden J, Chew A, Zorc JJ. Nebulized budesonide added to standard pediatric emergency department treatment of acute asthma: a randomized, double-blind trial. Acad Emerg Med. 2011;18:665–73.
Milani GKM, RosárioFilho NA, Riedi CA, Figueiredo BC. Nebulized budesonide to treat acute asthma in children. J Pediatr (Rio J). 2004;80:106–12.
Nuhoglu Y, Atas E, Nuhoglu C, Iscan M, Ozcay S. Acute effect of nebulized budesonide in asthmatic children. J Investig Allergol ClinImmunol. 2005;15:197–200.
Van Bever HP, Schuddinck L, Wojciechowski M, Stevens WJ. Aerosolized budesonide in asthmatic infants: a double blind study. Pediatr Pulmonol. 1990;9:177–80.
de Blic J, Delacourt C, Le Bourgeois M, Mahut B, Ostinelli J, Caswell C, et al. Efficacy of nebulized budesonide in treatment of severe infantile asthma: a double-blind study. J Allergy Clin Immunol. 1996;98:14–20.
Pedersen S, Hansen OR. Budesonide treatment of moderate and severe asthma in children: a dose-response study. J Allergy Clin Immunol. 1995;95:29–33.
Tsai YG, Lee MY, Yang KD, Chu DM, Yuh YS, Hung CH. A single dose of nebulized budesonide decreases exhaled nitric oxide in children with acute asthma. J Pediatr. 2001;139:433–7.
Akhtaruzzaman M, Ahmed SU, Hoque MA, Choudhury AM, Hossain MA, Islam MN, et al. Effects of nebulized budesonide as an adjunct to standard treatment of asthma exacerbations: a randomized, double-blind, placebo-controlled trial. Mymensingh Med J. 2014;23:418–25.
Barnes PJ. Inhaled corticosteroids. Pharmaceuticals (Basel). 2010;3:514–40.
Edmonds ML, Milan SJ, Camargo CA, Pollack CV, Rowe BH. Early use of inhaled corticosteroids in the emergency department treatment of acute asthma. Cochrane Database Syst Rev. 2012;12:CD02308.
Castro-Rodriguez JA, Pincheira MA, Escobar-Serna DP, Sossa-Briceño MP, Rodriguez-Martinez CE. Adding nebulized corticosteroids to systemic corticosteroids for acute asthma in children: a systematic review with meta-analysis. Pediatr Pulmonol. 2020;55:2508–17.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
CL designed the project; CL and ZL were involved in data collection and data analysis; CL prepared the manuscript; ZL edited the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not required for a systematic review.
Conflict of interest
There is no benefit has been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, CY., Liu, Z. Effect of budesonide on hospitalization rates among children with acute asthma attending paediatric emergency department: a systematic review and meta-analysis. World J Pediatr 17, 152–163 (2021). https://doi.org/10.1007/s12519-020-00403-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-020-00403-y