Abstract
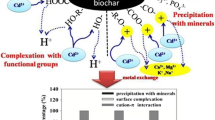
The objective of this study was to characterize the cadmium (Cd) adsorption behavior on biochar derived from grape and apple pruning residues (GPR and APR, respectively). Consequently, batch experiments were carried out with increasing levels of initial Cd concentration (0 to 200 mg L−1) under different shaking times (0 to 240 min) and temperatures (10, 20, 30, and 40 °C). The adsorption of Cd on the biochars was well fitted to the Elovich kinetics and Freundlich sorption model. The maximum Cd sorption capacities of 57 mg g−1 and 49 mg g−1 were calculated for GPR and APR biochars, respectively, indicating the higher sorption capacity of GPR in comparison with the APR biochar. The sorption energy parameter (E) of Dubinin–Radushkevich isotherm (4.37 and 4.05 kJ mol−1) and negative Gibbs free energy (∆G) values (− 15 to − 19 kJ mol−1) revealed the physical adsorption and spontaneous of Cd adsorption on the biochars, respectively. The entropy (ΔS) and change in enthalpy (ΔH) were found to be 1.57 J mol−1 K−1 and 0.22 kJ mol−1 for GPR biochar and 1.50 J mol−1 K−1 and 0.21 kJ mol−1 for APR biochar, reflecting an affinity of Cd on the biochars and endothermic nature of Cd adsorption reaction. This study demonstrated the feasibility of the biochars derived from grape and apple pruning residues to be as a potential low-cost adsorbent for Cd removal from aquatic systems. Furthermore, the adsorption procedure involving kinetic time and temperature effects were optimized to achieve the maximum Cd sorption on the adsorbent. Adsorption mostly took place within 20 and 40 min for GPR and APR biochar, respectively, while the optimum temperature was 40 °C.






Similar content being viewed by others
References
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36:327–363
Amouei AI, Amooey AA, Asgharzadeh F (2013) Cadmium removal from aqueous solution by canola residues: adsorption equilibrium and kinetics. Iran. Iran J Chem Chem Eng 10:39–50
ASTM D (2013) 84: Standard test method for chemical analysis of wood charcoal. American Society for Testing and Materials, West Conshohocken
Debnath S, Ghosh UC (2009) Nanostructured hydrous titanium (IV) oxide: synthesis, characterization and Ni (II) adsorption behavior. Chem Eng J 152:480–491
Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou Y, Zheng B, Cai X (2016) Competitive removal of Cd (II) and Pb (II) by biochars produced from water hyacinths: performance and mechanism. RSC Adv 6(7):5223–5232
Dume B, Mosissa T, Nebiyu A (2016) Effect of biochar on soil properties and lead (Pb) availability in a military camp in South West Ethiopia. Afr J Environ Sci Technol 10:77–85
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. T ASABE 51:2061–2069
Gueu S, Yao B, Adouby K, Ado G (2007) Kinetics and thermodynamics study of lead adsorption on to activated carbons from coconut and seed hull of the palm tree. Int J Environ Sci Technol 4:11–17
Han Y, Boateng AA, Qi PX, Lima IM, Chang J (2013) Heavy metal and phenol adsorptive properties of biochars from pyrolyzed switchgrass and woody biomass in correlation with surface properties. J Environ Manag 118:196–204
Hossain MA, Ngo HH, Guo WS, Setiadi T (2012) Adsorption and desorption of copper (II) ions onto garden grass. Bioresour Technol 121:386–395
Ibrahim MB, Sani S (2014) Comparative isotherms studies on adsorptive removal of Congo red from wastewater by watermelon rinds and neem-tree leaves. Open J Phys Chem 4:139–146
Juang RS, Chen ML (1997) Application of the Elovich equation to the kinetics of metal sorption with solvent-impregnated resins. Ind Eng Chem Res 36:813–820
Kılıc M, Kırbıyık C, Cepeliogullar Ö, Pütün A (2013) Adsorption of heavy metal ions from aqueous solutions by bio-char, a by-product of pyrolysis. Appl Surf Sci 283:856–862
Kumar PS, Ramalingam S, Sathyaselvabala V, Kirupha SD, Murugesan A, Sivanesan S (2012) Removal of cadmium (II) from aqueous solution by agricultural waste cashew nut shell. Korean. Korean J Chem Eng 29:756–768
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. J Waste Manag 28:215–225
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1367
Lee SJ, Park JH, Ahn YT, Chung JW (2015) Comparison of heavy metal adsorption by peat moss and peat moss-derived biochar produced under different carbonization conditions. Water Air Soil Pollut 226:1–11
Lu K, Yang X, Gielen G, Bolan N, Ok YS, Niazi NK, Xu S, Yuan G, Chen X, Zhang X, Liu D (2016) Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J Environ Manag 186:285–292
Ma F, Zhao B, Diao J (2016) Adsorption of cadmium by biochar produced from pyrolysis of corn stalk in aqueous solution. Water Sci Technol 74:1335–1345
Mohan D, Pittman CU Jr, Bricka M, Smith F, Yancey B, Mohammad J, Steele PH, Alexandre-Franco MF, Gomez-Serrano V, Gong H (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci 310:57–73
Nash JE, Sutcliffe JV (1970) River flow forecasting through conceptual models. Part 1: A discussion of principles. J Hydrol 10:282–290
Park JH, Choppala GK, Bolan NS, Chung JW, Chuasavathi T (2011) Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 348:439–451
Park JH, Ok YS, Kim SH, Cho JS, Heo JS, Delaune RD, Seo DC (2016) Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 142:77–83
Qi F, Yan Y, Lamb D, Naidu R, Bolan NS, Liu Y, Ok YS, Donne SW, Semple KT (2017) Thermal stability of biochar and its effects on cadmium sorption capacity. Bioresour Technol 246:48–56
Rao MM, Ramesh A, Rao GPC, Seshaiah K (2006) Removal of copper and cadmium from the aqueous solutions by activated carbon derived from Ceiba pentandra hulls. J Hazard Mater 129:123–129
Rostamian R, Heidarpour M, Mousavi SF, Afyuni M (2015) Characterization and sodium sorption capacity of biochar and activated carbon prepared from rice husk. J Agric Sci Technol 17:1057–1069
Sears GW Jr (1956) Determination of specific surface area of colloidal silica by titration with sodium hydroxide. Anal Chem 28:1981–1983
Sharma YC (2008) Thermodynamics of removal of cadmium by adsorption on indigenous clay. Chem Eng J 145:64–68
Singh B, Singh BP, Cowie AL (2010) Characterisation and evaluation of biochars for their application as a soil amendment. Soil Res 48:516–525
Smebye A, Alling V, Vogt RD, Gadmar TC, Mulder J, Cornelissen G, Hale SE (2016) Biochar amendment to soil changes dissolved organic matter content and composition. Chemosphere 142:100–105
Sun J, Lian F, Liu Z, Zhu L, Song Z (2014) Biochars derived from various crop straws: characterization and Cd (II) removal potential. Ecotoxicol Environ Saf 106:226–231
Sun K, Kang M, Ro KS, Libra JA, Zhao Y, Xing B (2016) Variation in sorption of propiconazole with biochars: the effect of temperature, mineral, molecular structure, and nano-porosity. Chemosphere 142:56–63
Taha MR, Ahmad K, Aziz AA, Chik Z (2009) Geoenvironmental aspects of tropical residual soils. In: Huat BBK et al (eds) Tropical residual soils engineering. Balkema, London, pp 377–403
Taiwo AF, Chinyere NJ (2016) Sorption characteristics for multiple adsorptions of heavy metal ions using activated carbon from Nigerian bamboo. J Mater Sci Chem Eng 4:39–49
Temkin MJ, Pyzhev V (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochim URSS 12:217–222
Tong X, Xu R (2013) Removal of Cu (II) from acidic electroplating effluent by biochars generated from crop straws. J Environ Sci 25:652–658
Webber TN, Chakravarti RK (1974) Pore and Solid Diffusion Models for fixed bed adsorbers. AIChE J 20:228–238
WHO (2008) World Health Organization Guidelines for Drinking Water Quality. Recommendations, World Health Organisation, Geneva
Yakkala K, Yu MR, Roh H, Yang JK, Chang YY (2013) Buffalo weed (Ambrosia trifida L. var. trifida) biochar for cadmium (II) and lead (II) adsorption in single and mixed system. Desalin Water Treat 51:7732–7745
Yang Y, Wang G, Wang B, Li Z, Jia X, Zhou Q, Zhao Y (2011) Biosorption of acid black and Congo Red from aqueous solution by nonviable penicillium YW 01: kinetic study, equilibrium isotherm and artificial neural network modelling. Bioresour Technol 102:828–834
Yang ZH, Xiong S, Wang B, Li Q, Yang WC (2013) Cr (III) adsorption by sugarcane pulp residue and biochar. J Cent S Univ Technol 20:1319–1325
Yang Y, Lin X, Wei B, Zhao Y, Wang J (2014) Evaluation of adsorption potential of bamboo biochar for metal-complex dye: equilibrium, kinetics and artificial neural network modelling. Int J Environ Sci Technol 11:1093–1100
Zhou Z, Lin S, Yue T, Lee T (2014) Adsorption of food dyes from aqueous solution by glutaraldehyde cross-linked magnetic chitosan nanoparticles. J Food Eng 126:133–141
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Hamzenejad Taghlidabad, R., Sepehr, E., Khodaverdiloo, H. et al. Characterization of cadmium adsorption on two cost-effective biochars for water treatment. Arab J Geosci 13, 448 (2020). https://doi.org/10.1007/s12517-020-05477-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-05477-6