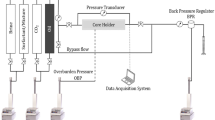
Abstract
Adsorption of surfactants on the reservoir rock is one the most important factors which decide the economic feasibility of recovery process. This study reports the adsorption of two in-house developed CO2-philic foaming surfactants on Malaysian sandstone. The point of zero charge (PZC) of the rock sample was determined to be 8.13. The surfactants contained single tail (FS-1) and two tails (FS-2) in their structures. Static adsorption tests were conducted as traditional bottle test as well as during the foam test experiments. For the bottle tests, it was noted that the FS-2 underwent more adsorption (4.94 mg/g) when compared to FS-1 (4.32 mg/g). The pH effect was significant, and at low pH value (pH 6), the adsorption was almost five times more for both surfactants when compared to the adsorption at high pH (pH 10) conditions. During the foam test at pH 6, the adsorption results for FS-1 and FS-2 were 4.02 and 4.48 mg/g, respectively. The effect of increasing the pH to 10 was comparable to bottle test results, and adsorption was decreased about five times. The bottle test gave slightly high adsorption values primarily because the contact time was longer (24 h) when compared to foaming test adsorption values where time is only about 30 minutes. In foaming tests, sparging of CO2 gas enhanced the shear mixing of contents thereby increasing the adsorption degree resulting in the similar adsorption values despite of less contact time. The study revealed that the adsorption of surfactant depends on many parameters, and one way of effective control is to adjust the pH of the fluid higher than the PZC of the rock.







Similar content being viewed by others
References
Ahmadi MA, Shadizadeh SR (2012) Adsorption of novel nonionic surfactant and particles mixture in carbonates: enhanced oil recovery implication. Energ Fuels 26:4655–4663
Ahmadi MA, Shadizadeh SR (2013a) Experimental investigation of adsorption of a new nonionic surfactant on carbonate minerals. Fuel 104:462–467
Ahmadi MA, Shadizadeh SR (2013b) Induced effect of adding nano silica on adsorption of a natural surfactant onto sandstone rock: experimental and theoretical study. J Petrol Sci Eng 112:239–247
Ahmadi MA, Zendehboudi S, Shafiei A, James L (2012) Nonionic surfactant for enhanced oil recovery from carbonates: adsorption kinetics and equilibrium. Ind Eng Chem Res 51:9894–9905
Appel C, Ma LQ, Dean Rhue R, Kennelley E (2003) Point of zero charge determination in soils and minerals via traditional methods and detection of electroacoustic mobility. Geoderma 113:77–93
Austad T, Rezaeidoust A, Puntervold T (2010) Chemical mechanism of low salinity water flooding in sandstone reservoirs. Paper presented at the SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA, 01/01/2010
Danov KD, Kralchevsky PA, Denkov ND, Ananthapadmanabhan KP, Lips A (2006) Mass transport in micellar surfactant solutions: 1. Relaxation of micelle concentration, aggregation number and polydispersity. Adv Colloid Interface Sci 119:1–16
Flaaten AK, Nguyen QP, Zhang J, Mohammadi H, Pope GA (2010) Alkaline/surfactant/polymer chemical flooding without the need for soft water. SPE J 15:184–196
Gao S (1994) Laboratory investigation of combination of alkaline-surfactant-polymer for Daqing EOR Society of Petroleum Engineers of AIME, (Paper) SPE:1–30
Grigg RB, Bai B, Liu Y (2004) Competitive adsorption of a hybrid surfactant system onto five minerals, berea sandstone, and limestone. Paper presented at the SPE Annual Technical Conference and Exhibition, Houston, Texas, 01/01/2004
Hussain S, Gul S, Khan S, Rehman H (2013) Retention studies of chromium (VI) from aqueous solution on the surface of a novel carbonaceous material. Arab J Geosci 6:4547–4556
Karnanda W, Benzagouta MS, AlQuraishi A, Amro MM (2013) Effect of temperature, pressure, salinity, and surfactant concentration on IFT for surfactant flooding optimization. Arab J Geosci 6:3535–3544
Lv W, Bazin B, Ma D, Liu Q, Han D, Wu K (2011) Static and dynamic adsorption of anionic and amphoteric surfactants with and without the presence of alkali. J Petrol Sci Eng 77:209–218
Manrique EJ, Thomas CP, Ravikiran R, Kamouei MI, Lantz M, Romero JL, Alvarado V (2010) EOR: current status and opportunities. Paper presented at the SPE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA, 24–28 April 2010
Mushtaq M, Tan IM, Ismail L, Lee SYC, Nadeem M, Sagir M (2013a) Oleate ester-derived nonionic surfactants: synthesis and cloud point behavior studies. J Dispersion Sci Technol 35:322–328
Mushtaq M, Tan IM, Ismail L, Nadeem M, Sagir M, Azam R, Hashmet R (2013b) Influence of PZC (point of zero charge) on the static adsorption of anionic surfactants on a Malaysian sandstone. J Dispersion Sci Technol. doi:10.1080/01932691.2013.785362
Mushtaq M, Tan IM, Ismail L, Nadeem M, Sagir M, Azam R, Hashmet R (2013c) Influence of PZC (point of zero charge) on the static adsorption of anionic surfactants on a Malaysian sandstone. J Dispersion Sci Technol 35:343–349
Paria S, Khilar KC (2004) A review on experimental studies of surfactant adsorption at the hydrophilic solid–water interface. Adv Colloid Interface Sci 110:75–95
Paria S, Manohar C, Khilar KC (2005) Adsorption of anionic and non-ionic surfactants on a cellulosic surface. Colloids Surf A 252:221–229
Rosen MJ (2004) Surfactants and interfacial phenomena. John Wiley & Sons, Inc
Sagir M, Tan IM, Mushtaq M, Ismail L, Nadeem M, Azam MR, Hashmet MR (2013) Novel surfactant for the reduction of CO2/brine interfacial tension. J Dispersion Sci Technol 35:463–470
Shafie N, Aris A, Puad N (2013) Influential factors on the levels of cation exchange capacity in sediment at Langat river. Arab J Geosci 6:3049–3058
Zendehboudi S, Ahmadi MA, Rajabzadeh AR, Mahinpey N, Chatzis I (2013) Experimental study on adsorption of a new surfactant onto carbonate reservoir samples—application to EOR. Can J Chem Eng 91:1439–1449
Zhang R, Somasundaran P (2006) Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv Colloid Interface Sci 123–126:213–229
Acknowledgments
The usage of EOR Research Centre UTP facilities is highly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mushtaq, M., Tan, I.M., Rashid, U. et al. Effect of pH on the static adsorption of foaming surfactants on Malaysian sandstone. Arab J Geosci 8, 8539–8548 (2015). https://doi.org/10.1007/s12517-014-1765-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12517-014-1765-4