Abstract
Background
Ever since the first description of hypertrophic cardiomyopathy (HCM), the most common genetic cardiac disease, tremendous progress has been made in the evaluation and management of HCM patients, but little attention has been focused on the impact of HCM on societal costs and quality of life (QoL).
Aims
This paper describes the study protocol for the AFFECT-HCM study into burden of disease (BoD), which aims to estimate health-related QoL and societal costs in HCM patients and genotype-positive phenotype-negative (G+/P−) relatives during a one-year follow-up study, and relate this to the phenotypical HCM expression.
Methods
A total of 400 Dutch HCM patients and 100 G+/P− subjects will be followed for one year in a prospective, multi-centre, prevalence-based BoD study. Societal costs will be measured via a bottom-up approach using the cost questionnaires iMCQ and iPCQ. For QoL, the generic EQ-5D-5L and disease-specific Kansas City Cardiomyopathy Questionnaire will be used. QoL and societal costs will be compared with phenotype-specific HCM characteristics and other determinants to identify factors that influence BoD. Accelerometry will test the correlation between BoD and physical activity.
Conclusion
The AFFECT-HCM study will evaluate the BoD in HCM patients and G+/P− subjects to improve the understanding of the societal and economic impact of HCM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
-
The AFFECT-HCM study will be the first Dutch study looking to quantify QoL and societal costs in HCM patients and G+/P− subjects.
-
It will provide novel insight into the influence of HCM on QoL perception and costs associated with HCM.
-
Physical activity will be tracked and possible correlations of physical activity in combination with HCM on QoL perception and costs will be investigated.
-
Important knowledge on the societal and economic impact of HCM will be acquired, which may assist in guiding future healthcare system and policy decisions, and help design economic evaluations.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common autosomal dominant genetic cardiac disease, affecting an estimated 1 in 200 to 500 people worldwide [1, 2]. It is characterised by left ventricular hypertrophy in the absence of abnormal loading conditions. It may cause symptoms and events such as dyspnoea, angina, palpitations, syncope or sudden cardiac death [3]. In the majority of cases, HCM is a monogenic disease and therefore family screening is recommended. Upon the clinical and, if applicable, genetic diagnosis of HCM, family members are advised to undergo pre-symptomatic genetic or cardiac screening [4]. This leads to the identification of family members carrying the familial pathogenic DNA variant without HCM (genotype-positive, phenotype-negative: G+/P−). These G+/P− subjects undergo regular cardiac evaluation in order to monitor possible HCM development. Thus, in addition to decreased physical, social and psychological functioning that HCM patients face, both HCM patients and G+/P− subjects may also face losses in quality of life (QoL) through external and internal stressors and anxiety [5, 6]. Consequently, HCM creates a social and economic burden for affected individuals and their relatives.
Burden of disease (BoD) studies measure the economic and societal impact of health conditions and gather valuable information for healthcare policy and decision-making [7]. Disease burden is often quantified in QoL expressed in utilities, as well as in societal costs captured in monetary units [8]. In addition to classical epidemiological measures and mortality, QoL and societal costs are increasingly recognised as central denominators of the disease burden [9]. However, within the current literature, not much research has been done investigating the QoL and societal costs in patients with HCM and G+/P− family members. Recent cost-of-illness studies have only estimated healthcare costs, leaving out information about broader cost types [10,11,12]. Furthermore, QoL studies are rare and none have measured QoL with the EQ-5D-5L, the required instrument according to Dutch guidelines [5, 13,14,15].
The AFFECT-HCM (Quality of Life and Costs in HCM) study aims to estimate the one-year impact of HCM on generic and disease-specific QoL and the societal costs of HCM in the Netherlands. The study will further explore the relationship between costs/QoL and phenotypic as well as socio-demographic characteristics. Moreover, physical activity in HCM patients and G+/P− subjects will be evaluated and linked to phenotype, QoL and cost outcomes. To our current knowledge, this will be the first BoD study performed in a Dutch setting that assesses broader cost types of HCM and the first BoD study that in addition to HCM patients also includes G+/P− relatives.
Methodology
Study design
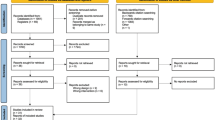
The AFFECT-HCM is a multi-centre prospective observational cohort study assessing the BoD of HCM patients and G+/P− relatives. Costs and QoL will be measured following a prevalence-based approach including subjects with various phenotypic expressions at different disease stages. Data will be collected at baseline and during follow-up after one year. Patients will be recruited from their respective hospitals for baseline analyses. The subject inclusion session will contain four questionnaires and clinical analyses consisting of a structured history and a physical examination. An electrocardiogram and echocardiography will be performed if these have not been performed within the last six months before the patient inclusion date. A physical activity tracker (ActiGraph), also known as an accelerometer, will be provided during two separate one-week intervals to monitor physical activity, assessing its possible influence and relationship on QoL perception and disease costs. A structured timeline is provided (Fig. 1).
Study population
Subjects will be divided into two main groups: phenotype-positive HCM patients and G+/P− relatives (Fig. 2). HCM patients must have undergone prior genetic testing and may be either G+ or genotype-negative (G−). HCM patients will be further subdivided into either non-obstructive or obstructive HCM (left ventricular outflow tract gradient of ≥30 mm Hg). Both of these subgroups will in turn be split into symptomatic (New York Health Association [NYHA] class II to IV) and asymptomatic patients (NYHA I).
Group and subgroup division of included subjects. Groups are divided into two main groups: genotype-positive phenotype-negative family relatives and hypertrophic cardiomyopathy (HCM) patients who have had prior genotyping. Patients are in turn divided into non-obstructive HCM and obstructive HCM. Both subgroups are further subdivided into symptomatic and asymptomatic patients
Recruitment
Genotyped HCM patients and G+/P− subjects aged 18–80 year will be recruited consecutively at dedicated cardio-genetic outpatient clinics in participating university hospitals that are part of the Double Dose research consortium and from cardiomyopathy databases in participating centres. Additionally, the Dutch patient advocacy group for HCM will distribute study information through their communication channels, allowing for patients from other hospitals to be included.
Inclusion criteria
-
Genotyped HCM patients
-
Or G+/P− family members
-
Ages 18–80 years old
-
Proficiency with the Dutch language
Exclusion criteria
-
Any subject not able to provide informed consent or fill in the questionnaires
Sample size
Due to the absence of sample size calculation methods for BoD studies, the sample size was based on previously conducted studies with similar designs indicating that a number of approximately 200 participants is sufficient [16,17,18]. Given the high prevalence of HCM, the low study burden for subjects, the number of participating academic HCM centres and the interest of carrying out subgroup analyses, a total number of 500 participants (consisting of approximately 400 HCM patients and 100 G+/P− carriers) is targeted and deemed feasible. As an underlying genetic variant can be found in 30–50% of the clinically diagnosed cases, the sample size of the G+ HCM group may be correspondingly smaller [19].
Data collection
Cost assessment
Costs will be estimated from a societal perspective including medical costs, patient and family costs, productivity losses, and costs outside the healthcare sector (Tab. 1). Therefore, corresponding healthcare resource utilisation of patients and relatives will be captured at an individual level via a bottom-up approach by using the iMCQ (medical consumption questionnaire) and the iPCQ (productivity cost questionnaire). The iMCQ entails 20 questions about the healthcare resource utilisations of patients for a broad range of healthcare services, while the iPCQ contains 12 questions about the lost productivity for paid and unpaid work. The monetary valuation of the used healthcare will be done with the Dutch costing tool by assigning reference prices to each resource [15]. For long-term productivity losses, the friction cost method will be used as recommended in the Dutch Manual for Costing [15]. As the study period does not exceed one year, discounting is not required.
Quality of life assessment
Generic QoL of patients and G+/P− relatives will be measured with the Dutch version of the standardised and validated EQ-5D-5L questionnaire as preferred by the Dutch guideline [15]. The EQ-5D-5L captures five health dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression), each with five answer levels resulting in 3125 possible health profiles [20]. These health profiles will be valued with the Dutch EQ-5D-5L value set in order to derive utility scores. Utilities are comparable between diseases and may be expressed in a score between zero (death) and one (perfect health). After a year, quality-adjusted life years (QALYs) can be derived as this measure combines the quality of life (utility) with the length of life [15, 21].
Disease-specific QoL will be measured with the Kansas City Cardiomyopathy Questionnaire (KCCQ), which covers seven health domains (symptom frequency, symptom burden, symptom stability, physical limitations, social limitations, quality of life, and patient self-efficacy) [22]. All dimensions range from zero (worst functioning) to 100 (excellent health). The KCCQ has been proven reliable and sensitive to monitor clinical status, and has been validated for HCM [13, 23, 24].
Physical activity measurement
The ActiGraph accelerometer (Model wGT3X-BT; ActiGraph, LLC., Pensacola, Florida, USA) will be used to objectively measure the volume of physical activity of each subject. An ActiGraph accelerometer is designed to detect body movements. It has been validated for medical research in adults [25, 26]. The accelerometer will be worn for two separate one-week periods (worn on a belt around the hip), except when bathing.
Outcomes
Primary outcomes
-
Societal costs of HCM and G+/P− status will be adjusted for inflation and expressed in euro of the respective reporting year. Baseline costs will be extrapolated to one year and described as costs per patient per year. After study completion, longitudinal cost data will be analysed in order to observe potential cost developments or trends.
-
Generic QoL will be reported in utilities and disease-specific QoL will be shown on a 0–100 scale. QALYs will be calculated and reported.
Secondary outcomes
-
The potential relationship between costs, QoL and phenotypic as well as socio-demographic characteristics will be analysed with correlation and regression techniques.
-
Potential differences in costs and QoL between phenotype status (HCM vs G+/P− subjects) and disease subtypes (non-obstructive HCM vs obstructive HCM), and therein symptomatic versus asymptomatic patients, will be explored with subgroup analyses (Tab. 2).
-
Physical activity between the subgroups will be evaluated by accelerometry, which will be linked to QoL and cost outcomes.
Statistical analysis
Statistical analysis will be performed with baseline patient characteristics at one year and with longitudinal data after follow-up of the last patient following finalisation of the study. We will use IBM SPSS Statistics version 25 (SPSS, IBM, Armonk, New York). Patient characteristics and baseline outcomes will be analysed with descriptive methods by providing relative frequencies for categorical data, mean and standard deviations for normally distributed continuous data, or median and interquartile ranges in case of non-normality. Normality of data will be tested using the Shapiro–Wilk test. As the cost-distribution is expected to be non-normal, bootstrapping (1000 simulations) will be performed to calculate 95% confidence intervals [27]. Missing data will be considered irrelevant for further analysis and excluded.
Potential correlations between costs and other variables, such as age or disease severity, will be analysed using the non-parametric Kendall’s tau‑b correlation coefficient. Furthermore, we will use multiple regression to identify variables that are associated with QoL. Moreover, subgroup differences in costs and QoL will be analysed with the chi-squared (χ2) test for categorical data, the independent samples t‑test for normally distributed continuous data, and the Mann-Whitney U test for non-normally distributed continuous data, and will be reported for each subgroup respectively. The planned subgroup analyses are summarised in Tab. 2. For accelerometry measurements, the activity intensity, i.e. the number of minutes per hour of moderate to vigorous physical activity (> 3 metabolic equivalents), will be calculated using a threshold value of 2100 counts per minute [26]. Activity counts per hour shall be modified to counts per minute to provide a measure of activity volume, which allows for comparison with other studies. Between-group results will be compared using a two-way analysis of variance to test for main effects between the groups. Furthermore, one-way sensitivity analyses will be performed. Base case assumptions for cost prices will be varied to analyse their impact on the results. Lastly, costs will also be calculated from a healthcare perspective.
Ethics
The study will be conducted according to the principles of the Declaration of Helsinki and the ISO 14155 Good Clinical Practice guidelines for medical devices. The study does not fall under the scope of the Medical Research Involving Human Subjects Act and has been approved by the Institutional Review Board of the Erasmus University Medical Centre Rotterdam (MEC-2022-0036).
Discussion
The AFFECT-HCM study will be the first Dutch BoD study of HCM. It sets out to measure QoL and societal costs in HCM patients and G+/P− subjects during a one-year follow-up period. Due to the clinical heterogeneity of HCM, the disease burden is assumed to vary between the previously defined subgroups. Furthermore, this study is expected to provide valuable insights into QoL perception and mental wellbeing of G+/P− variant carriers. This is important since carriers may fear developing HCM with its associated health consequences, something which may have been observed in affected family members. The study will also collect novel information about the economic burden of HCM, in addition to evaluating costs beyond healthcare, as these broader costs are considerably less explored.
The AFFECT-HCM study has some limitations. Firstly, the planned bottom-up approach will use patient-reported outcomes, i.e. data based on information given by participants. This might be subjective and may sometimes be based on estimations. However, previous cost-of-illness studies used aggregated databases and thus neglected important information about broader costs such as patient and family costs, productivity losses and costs beyond healthcare, which are included in this study [10,11,12]. Secondly, the to-be-used questionnaires are validated for an adult population only, hence minors will be excluded [20]. Thirdly, potential recall-bias due to the retrospective measurement of healthcare resources may take place. In order to minimise potential recall-bias, whilst ensuring a high informative value, a recall-period of three months is regarded as optimal [28].
This study protocol will be the first Dutch prospective cohort study in HCM patients and G+/P− carriers to provide insights into different methods for estimating the BoD, which could provide a framework to guide future research. It will also provide important patient-specific information for economic evaluations to analyse different care strategies and to further optimise care for HCM patients. To improve the understanding of the disease impact, future research into the BoD in HCM should be performed in more settings.
References
Maron BJ, Gardin JM, Flack JM, et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation. 1995;92:785–9.
Zou Y, Song L, Wang Z, et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am J Med. 2004;116:14–8.
Maron BJ. Hypertrophic CardiomyopathyA Systematic Review. JAMA. 2002;287:1308–20.
van Langen IM, Hofman N, Tan HL, et al. Family and population strategies for screening and counselling of inherited cardiac arrhythmias. Ann Med. 2004;36:116–24.
Cox S, O’Donoghue AC, McKenna WJ, et al. Health related quality of life and psychological wellbeing in patients with hypertrophic cardiomyopathy. Heart. 1997;78:182–7.
Morgan JF, O’Donoghue AC, McKenna WJ, et al. Psychiatric disorders in hypertrophic cardiomyopathy. Gen Hosp Psychiatry. 2008;30:49–54.
Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol. 2014;20:327–37.
Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the Economic Evaluation of Health Care Programmes. 4th ed. Oxford: Oxford University Press; 2015.
Hyder AA, Puvanachandra P, Morrow RH. Measuring the health of populations: explaining composite indicators. J Public Health Res. 2012;1:222.
Butzner M, Maron M, Sarocco P, et al. Healthcare resource utilization and cost of obstructive hypertrophic cardiomyopathy in a US population. Cardiol Res Pract. 2022;13:100089.
Jain SS, Li SS, Xie J, et al. Clinical and economic burden of obstructive hypertrophic cardiomyopathy in the United States. J Med Econ. 2021;24:1115–23.
Jan A, Shah MA, Rehman S. Hypertrophic Obstructive Cardiomyopathy and the Cost of Treatment. Am J Cardiol. 2016;117:S4.
Capota R, Militaru S, Ionescu AA, et al. Quality of life status determinants in hypertrophic cardiomyopathy as evaluated by the Kansas City Cardiomyopathy Questionnaire. Health Qual Life Outcomes. 2020;18:1–8.
Christiaans I, van Langen IM, Birnie E, et al. Quality of life and psychological distress in hypertrophic cardiomyopathy mutation carriers: a cross-sectional cohort study. Am J Med Genet A. 2009;149:602–12.
Hakkaart-van Roijen L, van der Linden N, Bouwmans C, et al. Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. (Methodology for cost analysis and price references for economical evaluations in healthcare.). Available in Dutch: Zorginstituut Nederland; 2015.
Johnston KM, Lakzadeh P, Donato BM, et al. Methods of sample size calculation in descriptive retrospective burden of illness studies. Bmc Med Res Methodol. 2019;19:1–7.
Dahham J, Rizk R, Hiligsmann M, et al. The Economic and societal burden of multiple sclerosis on lebanese society: a cost-of-illness and quality of life study protocol. Expert Rev Pharmacoecon Outcomes Res. 2022;22:869–76.
Delgado JF, Oliva J, Llano M, et al. Health care and nonhealth care costs in the treatment of patients with symptomatic chronic heart failure in Spain. Rev Esp Cardiol (English Ed.) 2014;67:643–50.
Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and evaluation of hypertrophic cardiomyopathy: JACC state-of-the-art review. Am J Cardiol. 2022;79:372–89.
Versteegh MM, Vermeulen KM, Evers SM, et al. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19:343–52.
Busschbach JV, van Hout BA, de Wit A. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg, bijlage 2: QALY en kwaliteit van leven metingen. (Guideline for conducting economic evaluations in health care, attachment 2: QALY and quality of life measurements.) Zorginstituut Ned, Diemen. 2016 Feb 29 [cited 2022 Aug 1]. Available in Dutch. Available from: https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg.
Spertus JA, Jones PG, Sandhu AT, et al. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. Am J Cardiol. 2020;76:2379–90.
Nassif M, Fine JT, Dolan C, et al. Validation of the Kansas City Cardiomyopathy Questionnaire in symptomatic obstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2022;10:531–9.
Rodríguez-Artalejo F, Guallar-Castillón P, Pascual CR, et al. Health-related quality of life as a predictor of hospital readmission and death among patients with heart failure. Arch Intern Med Res. 2005;165:1274–9.
Howell J, Delisle S, Jones M, et al. Actigraphy in heart failure subjects with a reduced and normal ejection fraction. J Card Fail. 2008;14:S110.
Loprinzi PD, Lee H, Cardinal BJ, et al. The relationship of actigraph accelerometer cut-points for estimating physical activity with selected health outcomes: results from NHANES 2003–06. Res Q Exerc Sport. 2012;83:422–30.
Mooney CZ, Mooney CF, Mooney CL, et al. Bootstrapping: A nonparametric approach to statistical inference. SAGE; 1993.
Kjellsson G, Clarke P, Gerdtham U‑G. Forgetting to remember or remembering to forget: a study of the recall period length in health care survey questions. J Health Econ. 2014;35:34–46.
Acknowledgements
This paper is part of the project ‘Double Dose of energy and efforts of the national DOSIS consortium to design and test new diagnostic and treatment strategies for inherited cardiomyopathies (DOUBLE DOSE)’, which is funded by the Dutch Heart Foundation and the Dutch Foundation (Stichting) Hartedroom (Grant number: 2020B005).
Funding
This study is supported by Bristol Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. A. C. Schoonvelde, I. Wiethoff, M. Hiligsmann, S. M. A. A. Evers and M. Michels declare that they have no competing interests.
Additional information
The authors Stephan A. C. Schoonvelde and Isabell Wiethoff contributed equally to the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schoonvelde, S.A.C., Wiethoff, I., Hiligsmann, M. et al. Quality of life and societal costs in hypertrophic cardiomyopathy: protocol of the AFFECT-HCM study. Neth Heart J 31, 238–243 (2023). https://doi.org/10.1007/s12471-022-01753-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12471-022-01753-0