Abstract
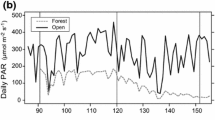
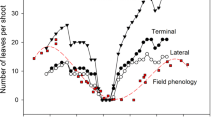
Spring leaf flush and changes in the understory radiation in montane deciduous forests are major determinants of the life cycle of spring ephemerals, which complete their epigeous growth before canopy closure in spring. We compared the growth, carbon allocation, and reproduction of a spring ephemeral, Erythronium japonicum (Balrer) Decne., between the ambient (control) and early shade treatments in the field during the flowering (early-May), fruiting (mid- May), and fruit ripening periods (late-May), under the assumption of early spring canopy closure due to climate change. Carbon allocation was investigated using a 13C labelling experiment. Both sterile (non-flowering) and fertile (flowering) E. japonicum under the shade treatment showed higher specific leaf area and earlier leaf senescence than those in the ambient conditions (p < 0.05). The flowering E. japonicum concentrated on biomass allocation to the aboveground vegetative organs prior to fruiting and to reproductive and storage organs from fruiting. E. japonicum used carbohydrates stored during the previous year for vegetative growth, while the current-year photosynthates were used for the current-year reproduction. Carbon allocation to fruit began earlier under the shade treatment, demonstrating that E. japonicum allocated the current-year photosynthates more to reproduction than to vegetative organs under the early shade conditions. However, the seed size (p = 0.012) and germination rate (p = 0.008) were significantly lower under the shade treatment than under the ambient conditions, implying a potential decrease in viable seed production in the shorter high-light period. The earlier leaf flush could be a critical threat to the population maintenance of spring ephemerals such as E. japonicum.
Similar content being viewed by others
References
Augspurger CK (1984) Light requirements of neotropical tree seedlings: a comparative study of growth and survival. J Ecol 72:777–795
Beaubien E, Freeland H (2000) Spring phenology trends in Alberta, Canada: links to ocean temperature. Int J Biometeorol 44:53–59
Benvenuti S, Macchia M, Stefani A (1994) Effects of shade on reproduction and some morphological characteristics of Abutilon theophrasti Medicos, Datura stramonium L. and Sorghum halepense L. Pers. Weed Res 34:283–288.
Burd M (1994) Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot Rev 60:83–139
Blodgett FH (1910) The origin and development of bulbs in the genus Erythronium. Botanical gazette 50:340–373
Dale MP, Causton DR (1992) The ecophysiology of Veronica Chamaedrys, V. Montana and V. Officinalis. III. effects of shading on the phenology of biomass allocations — a field experiment. J Ecol 80:505–515
Easu K (1965) Plant Anatomy. John Wiley & Sons, Inc. New York
Eickmeier WG, Schussler EE (1993) Responses of the spring ephemeral Claytonia virginica L. to light and nutrient manipulations and implications for the “vernal-dam” hypothesis. Bull Torrey Bot Club 120:157–165
Fitter AH, Fitter RSR (2002) Rapid changes in flowering time in British plants. Science 296:1689–1691
Gandin A, Dizengremel P, Lapointe L (2011) Photoperiod has a stronger impact than irradiance on the source–sink relationships in the sink-limited species Erythronium americanum. Botany 89:763–770
García MB, Ehrlén J (2002) Reproductive effort and herbivory timing in a perennial herb: fitness components at the individual and population levels. Am J Bot 89:1295–1302
Ghering JL, Delph LF (2006) Effects of reduced source-sink ratio on the cost of reproduction in females of Silene latifolia. J Plant Sci 167:843–851
Hasegawa S, Koba K, Tayasu I, Takeda H, Haga H (2003) Carbon autonomy of reproductive shoots of Siberian alder (Alnus hirsuta var. sibirica). J Plant Res 116:18–188
Horibata S, Hasegawa SF, Kudo G (2007) Cost of reproduction in a spring ephemeral species, Adonis ramosa (Ranunculaceae): carbon budget for seed production. Ann Bot 100:565–571
Humbeck K (2013) Epigenetic and small RNA regulation of senescence. Plant Mol Biol 82:529–537
Ida TY, Kudo G (2008) Timing of canopy closure influences carbon translocation and seed production of an understorey herb, Trillium apetalon (Trilliaceae). Ann Bot 101:435–446
ISTA (2010) International Rules for Seed Testing. International Seed Testing Association, Bassersdorf, Switzerland
Kawano S (2005) Life-history monographs of Japanese plants. 1: Erythronium japonicum Decne. (Liliaceae). Plant Spec Biol 20:67–74
Kawano S, Hiratsuka A, Hayashi K (1982) Life history characteristics and survivorship of Erythronium Japonicum. The productive and reproductive biology of flowering plants V. Oikos 38:129–149
Kim SY, Lee SY, Rhie YH, Kim KS (2014) Breaking bud dormancy in Erythronium japonicum Decne. (Liliaceae) by natural and artificial chilling. Hort Environ Biotechnol 55:380–386
Kitaoka S, Watanabe Y, Koike T (2009) The effects of cleared larch canopy and nitrogen supply on gas exchange and leaf traits in deciduous broad-leaved tree seedlings. Tree Physiol 29:1503–1511
Korea Meteorological Administration (2014) Weather information. Available at: http://www.kma.go.kr/weather/climate/average_ 30years.jsp (accessed on 8 January 2014)
Korea National Arboretum (2011) Reports on the conservation and adaptation projects for the forest species sensitive to climate change. Korea National Arboretum, Pocheon
Lambers H, Chapin III FS, Pons TL (2008) Plant Physiological Ecology, 2nd ed. Springer, New York.
Lapointe L (2001) How phenology influences physiology in deciduous forest spring ephemerals. Physiol Plantarum 113:151–157
Larcher W (2003) Physiological Plant Ecology: Ecophysiology and Stress Physiology of Function Groups, 4th ed. Springer-Verlag Berlin Heidelberg
Lichtenthaler HK, Buschmann C, Doll M, Fietz H, Bach T, Kozel U, Meier D, Rahmsdorf U (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res 2:115–141
Lubbers AE, Christensen NL (1986) Intraseasonal variation in seed production among flowers and plants of Thalictrum thalictroides (Ranunculaceae). Am J Bot 73:190–203
Lubbers AE, Lechowicz MJ (1989) Effects of leaf removal on reproductions vs. belowground storage in Trillium grandiflorum. Ecology 70:85–96
McKenna MF, Houle G (2000) Why are annual plants rarely spring ephemerals? New Phytol 148:295–302
McKenna, MF (1999) The effect of The effect of light on the growth and reproduction of Floerkea proserpinacoides. New Phytol 141:99–108
Menzel A (2000) Trends in phenological phases in Europe between 1951 and 1996. Int J Biometeorol 44:76–81
Menzel A, Sparks TH, Estrella N et al. (2006) European phenological response to climate change matches the warming pattern. Glob Change Biol 12:1969–1976
Muller RN (1978) The phenology, growth and ecosystem dynamics of Erythronium americanum in the northern hardwood forest. Ecol Monogr 48:1–20
National Academy of Agricultural Science, 2011. Korea Soil Classification and Explanation. National Academy of Agricultural Science, Rural Development Administration, Suwon
Nishikawa Y (2009) Significance of intra-inflorescence variation on flowering time of a spring ephemeral, Gagea lutea (Liliaceae), under seasonal fluctuations of pollinator and light availabilities. Plant Ecol 202:337–347
Park YM, Park PS, Sohng JE, Lee SK, Kim M-J (2010) Changes in growth and reproductive strategy of Disporum smilacinum in canopy gap and closed canopy areas. Hort Environ Biotechnol 51:463–469
Risser P, Cottam G (1967) Influence of temperature on the dormancy of some spring ephemerals. Ecology 48:500–503
Routhier MC, Lapointe L (2002) Impact of tree leaf phenology on growth rates and reproduction in the spring flowering species Trillium erectum (Liliaceae). Am J Bot 89:500–505
Schemske DW, Willson MF, Melampy MN, Miller LJ, Verner L, Schemske KM, Best LB (1978) Flowering ecology of some spring woodland herbs. Ecology 59:351–366
Sparling J (1967) Assimilation rates of some woodland herbs in Ontario. Bot Gaz 128:160–168
Sunmonu N, Kudo G (2014) How do sink and source activities influence the reproduction and vegetative growth of spring ephemeral herbs under different light conditions? J Plant Res 127:503–511
Vézina PE, Grandtner MM (1965) Phenological observations of spring geophytes in Quebec. Ecology 46:869–872
Vitasse Y, Porté AJ, Kremer A, Michalet R, Delzon S (2009) Responses of canopy duration to temperature changes in four temperate tree species, relative contributions of spring and autumn leaf phenology. Oecologia 161:187–198
Yokoi Y (1976) Growth and reproduction in higher plants II. Analytical study of growth and reproduction of Erythronium japonicum. J Plant Res 89:15–31
Yoshie F, Fukuda T (1994) Effects of growth temperature and winter duration on leaf phenology of Erythronium japonicum, a forest spring geophyte. Oecologia 97:366–368
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H.J., Jung, J.B., Jang, Y.L. et al. Effects of experimental early canopy closure on the growth and reproduction of spring ephemeral Erythronium japonicum in a montane deciduous forest. J. Plant Biol. 58, 164–174 (2015). https://doi.org/10.1007/s12374-014-0545-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-014-0545-8