Abstract
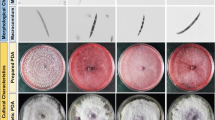
Productivity in sugarcane, a vegetatively propagated crop, is affected by various diseases. Red rot caused by the fungal pathogen Colletotrichum falcatum Went is a devastating disease of sugarcane causing serious yield losses in India and in many other countries. Understanding the pathogenesis of this hemibiotrophic fungal pathogen within host tissue will deepen our knowledge on host–pathogen interaction and helps to develop effective disease management strategies. Green fluorescent protein (GFP) is an effective tool for exploring fungal biology during host–pathogen interactions. In the present study we have conducted detailed investigations using GFP transformed strain of C. falcatum to establish the pathogenesis, colonization and spread of this fungus inside host tissues. We transformed C. falcatum with eGFP using Agrobacterium-mediated transformation using hygromycin B as a selectable marker and the transgene was stably integrated with mitotic stability. The C. falcatum transformants maintained morphological characters and growth parameters as like the wild type and the virulence nature was not altered as compared to the wild-type C. falcatum. GFP-tagged C. falcatum pathotypes clearly demonstrated the variations in C. falcatum colonization during compatible and incompatible interactions with sugarcane. These variations were comparable to in planta studies conducted using non GFP-tagged C. falcatum and using calcofluor staining. This was further validated with histological assays with calcofluor staining. The study is first of its kind in sugarcane and C. falcatum and will give a better understanding of pathogenesis inside the host in a spatio-temporal manner.








Similar content being viewed by others
References
Ashwin, N.M.R., L. Barnabas, A.R. Sundar, P. Malathi, R. Viswanathan, A. Masi, G.K. Agrawal, and R. Rakwal. 2017. Comparative secretome analysis of Colletotrichum falcatum identifies a cerato-platanin protein (EPL1) as a potential pathogen-associated molecular pattern (PAMP) inducing systemic resistance in sugarcane. Journal of Proteomics 169: 2–20.
Bergstrom, G.C., and R.L. Nicholson. 1999. The biology of corn anthracnose: knowledge to exploit for improved management. Plant Disease 83: 596–608.
Buron-Moles, G., M. López-Pérez, L. González-Candelas, I. Viñas, N. Teixidó, J. Usall, and R. Torres. 2012. Use of GFP-tagged strains of Penicillium digitatum and Penicillium expansum to study host–pathogen interactions in oranges and ales. International Journal of Food Microbiology 160: 162–170.
Chen, N., T. Hsiang, and P.H. Goodwin. 2003. Use of green fluorescent protein to quantify the growth of Colletotrichum during infection of tobacco. Journal of Microbiological Methods 53: 113–122.
De Groot, M.J., P. Bundock, P.J. Hooykaas, and A.G. Beijersbergen. 1998. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nature Biotechnology 16(9): 839.
Flowers, J.L., and L.J. Vaillancourt. 2005. Parameters affecting the efficiency of Agrobacterium tumefaciens-mediated transformation of Colletotrichum graminicola. Current Genetics 48: 380–388.
Honeycutt, R.J., B.W. Sobral, P. Keim, and J.E. Irvine. 1992. A rapid DNA extraction method for sugarcane and its relatives. Plant Molecular Biology Reporter 10(1): 66–72.
Hsu, J. 1996. Multiple comparisons: Theory and methods. London: Chapman and Hall.
Kaverinathan, K., M. Scindiya, P. Malathi, R. Viswanathan, and A.R. Sundar. 2017. Role of melanin in Colletotrichum falcatum pathogenesis causing sugarcane red rot. Sugar Tech 19: 584–591.
Li, C., S. Chen, C. Zuo, Q. Sun, Q. Ye, G. Yi, and B. Huang. 2011. The use of GFP-transformed isolates to study infection of banana with Fusarium oxysporum f. sp. cubense race 4. European Journal of Plant Pathology 131: 327–340.
Lorang, J.M., R.P. Tuori, J.P. Martinez, T.L. Sawyer, R.S. Redman, J.A. Rollins, T.J. Wolpert, K.B. Johnson, R.J. Rodriguez, M.B. Dickman, and L.M. Ciuffetti. 2001. Green fluorescent protein is lighting up fungal biology. Applied and Environmental Microbiology 67: 1987–1994.
Maor, R., M. Puyesky, B.A. Horwitz, and A. Sharon. 1998. Use of green fluorescent protein (GFP) for studying development and fungal–plant interaction in Cochliobolus heterostrophus. Mycological Research 102: 491–496.
Maruthachalam, K., N.V. Nair, H.S. Rho, J. Choi, S. Kim, and Y.H. Lee. 2008. Agrobacterium tumefaciens-mediated transformation in Colletotrichum falcatum and C. acutatum. Journal of Microbiology and Biotechnology 18: 234–241.
Michielse, C.B., P.J. Hooykaas, C.A. van den Hondel, and A.F. Ram. 2005. Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Current Genetics 48: 1–17.
Muench, S., N. Ludwig, D.S. Floss, J.A. Sugui, A.M. Koszucka, L.M. Voll, U.W.E. Sonnewald, and H.B. Deising. 2011. Identification of virulence genes in the corn pathogen Colletotrichum graminicola by Agrobacterium tumefaciens-mediated transformation. Molecular Plant Pathology 12: 43–55.
Mullins, E.D., X. Chen, P. Romaine, R. Raina, D.M. Geiser, and S. Kang. 2001. Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology 91: 173–180.
O’Connell, R., C. Herbert, S. Sreenivasaprasad, M. Khatib, M.T. Esquerré-Tugayé, and B. Dumas. 2004. A novel Arabidopsis-Colletotrichum pathosystem for the molecular dissection of plant–fungal interactions. Molecular Plant–Microbe Interactions 17: 272–282.
Park, S.Y., M.H. Jeong, H.Y. Wang, J.A. Kim, N.H. Yu, S. Kim, Y.H. Cheong, S. Kang, Y.H. Lee, and J.S. Hur. 2013. Agrobacterium tumefaciens-mediated transformation of the lichen fungus, Umbilicaria muehlenbergii. PLoS ONE 8(12): e83896.
Perfect, S.E., H.B. Hughes, R.J. O’Connell, and J.R. Green. 1999. Colletotrichum: a model genus for studies on pathology and fungal–plant interactions. Fungal Genetics and Biology 27: 186–198.
Pliego, C., S. Kanematsu, D. Ruano-Rosa, A. De Vicente, C. López-Herrera, F.M. Cazorla, and C. Ramos. 2009. GFP sheds light on the infection process of avocado roots by Rosellinia necatrix. Fungal Genetics and Biology 46: 137–145.
Prasanth, C.N., R. Viswanathan, N. Krishna, P. Malathi, A.R. Sundar, and T. Tiwari. 2017. Unravelling the genetic complexities in gene set of sugarcane red rot pathogen Colletotrichum falcatum through transcriptomic approach. Sugar Tech 19: 604–615.
Prasher, D.C., V.K. Eckenrode, W.W. Ward, F.G. Prendergast, and M.J. Cormier. 1992. Primary structure of the Aequorea victoria green-fluorescent protein. Gene 111: 229–233.
Rahul, P.R., V. Ganesh Kumar, R. Viswanathan, A. Ramesh Sundar, P. Malathi, C.N. Prasanth, and P.T. Prathima. 2016. Defense transcriptome analysis of sugarcane and Colletotrichum falcatum interaction using host suspension cells and pathogen elicitor. Sugar Tech 18: 16–28.
Ruiz-Díez, B. 2002. Strategies for the transformation of filamentous fungi. Journal of Applied Microbiology 92: 189–195.
Sathyabhama, M., R. Viswanathan, M. Nandakumar, P. Malathi, and A.R. Sundar. 2015. Understanding sugarcane defence responses during the initial phase of Colletotrichum falcatum pathogenesis by suppression subtractive hybridization (SSH). Physiological and Molecular Plant Pathology 91: 131–140.
Spellig, T., A. Bottin, and R. Kahmann. 1996. Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis. Molecular and General Genetics 252: 503–509.
Srinivasan, K.V., and N.R. Bhat. 1961. Red rot of sugarcane—Criteria for grading resistance. Journal of Indian Botanical Society 40: 566–577.
Sundara, B. 1998. Sugarcane cultivation. New Delhi: Vikas Publishers.
van Kan, J.A., G.F.J.M. Van den Ackerveken, and P.J.G.M. De Wit. 1991. Cloning and characterization of cDNA of avirulence gene avr9 of the fungal pathogen Cladosporium fulvum, causal agent of tomato leaf mold. Molecular Plant–Microbe Interaction 4: 52–59.
van West, P., B. Reid, T.A. Campbell, R.W. Sandrock, W.E. Fry, S. Kamoun, and N.A. Gow. 1999. Green fluorescent protein (GFP) as a reporter gene for the plant pathogenic oomycete Phytophthora palmivora. FEMS Microbiology Letters 178: 71–80.
Viswanathan, R. 2010. Plant disease: Red rot of sugarcane. New Delhi: Anmol Publications.
Viswanathan, R. 2012. Molecular basis of red rot resistance in sugarcane. In Functional plant science and biotechnology 6 (special issue 2), sugarcane pathology, ed. R. Viswanathan and A.R. Sundar, 40–50. Ikenobe: Global Science Books.
Viswanathan, R. 2018. Changing scenario of sugarcane diseases in India since introduction of hybrid cane varieties: Path travelled for a century. Journal of Sugarcane Research 8(1): 1–35.
Viswanathan, R., P. Malathi, and P. Padmanaban. 2003. Variation in sugarcane red rot pathogen Colletotrichum falcatum Went. In Frontiers of fungal diversity in India, ed. G.P. Rao, C. Manoharachari, D.J. Bhat, R.C. Rajak, and T.N. Lakhanpal, 639–667. Lucknow: International Book Distributing Co.
Viswanathan, R., P. Padmanaban, and D. Mohanraj. 1997. Growing virulence of red rot pathogen of sugarcane in Tamil Nadu. Indian Sugar 47(1): 23–30.
Viswanathan, R., A.R. Sundar, R. Selvakumar, and P. Malathi. 2018. Progress in understanding fungal diseases affecting sugarcane: red rot. In Achieving sustainable cultivation of sugarcane volume 2: Breeding, pests and diseases, ed. P. Rott, 201–220. Cambridge: Burleigh Dodds Science Publishing. https://doi.org/10.19103/AS.2017.0035.21.
Viswanathan, R., and R. Samiyappan. 1999. Red rot disease in sugarcane: A major constraint for the Indian sugar industry. Sugar Cane 5: 9–15.
Viswanathan, R., M. Sathyabhama, P. Malathi, and A.R. Sundar. 2016. Transcriptome analysis of host–pathogen interaction between sugarcane and Colletotrichum falcatum by suppression subtractive hybridization and Illumina sequencing. Proceedings of the International Society of Sugar Cane Technologists 29: 1639–1644.
Zhang, W.W., T.F. Jiang, X. Cui, F.J. Qi, and G.L. Jian. 2013. Colonization in cotton plants by a green fluorescent protein labelled strain of Verticillium dahliae. European Journal of Plant Pathology 135: 867–876.
Acknowledgements
The authors are grateful to the Directors of the Institute for providing facilities for the research work. The financial support received from Sugarcane Development Fund (No. 6-7/2006-R&D/SDF/885), Ministry of Consumer Affairs, Food and Public Distribution, New Delhi, is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nandakumar, M., Malathi, P., Sundar, A.R. et al. Use of Green Fluorescent Protein Expressing Colletotrichum falcatum, the Red Rot Pathogen for Precise Host–Pathogen Interaction Studies in Sugarcane. Sugar Tech 22, 112–121 (2020). https://doi.org/10.1007/s12355-019-00751-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00751-8