Abstract
Background
We aimed to compare coronary artery calcium scoring (CACS) with computed tomography (CT) with 80 and 120 kVp in a large patient population and to establish whether there is a difference in risk classification between the two scores.
Methods
Patients with suspected CAD undergoing MPS were included. All underwent standard CACS assessment with 120-kVp tube voltage and with 80 kVp. Two datasets (low-dose and standard) were generated and compared. Risk classes (0 to 25, 25 to 50, 50 to 75, 75 to 90, and > 90%) were recorded.
Results
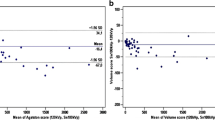
1511 patients were included (793 males, age 69 ± 9.1 years). There was a very good correlation between scores calculated with 120 and 80 kVp (R = 0.94, R2 = 0.88, P < .001), with Bland–Altman limits of agreement of − 563.5 to 871.9 and a bias of − 154.2. The proportion of patients assigned to the < 25% percentile class (P = .03) and with CACS = 0 differed between the two protocols (n = 264 vs 437, P < .001).
Conclusion
In a large patient population, despite a good correlation between CACS calculated with standard and low-dose CT, there is a systematic underestimation of CACS with the low-dose protocol. This may have an impact especially on the prognostic value of the calcium score, and the established “power of zero” may no longer be warranted if CACS is assessed with low-dose CT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery calcium score (CACS) is easily assessed noninvasively by cardiac computed tomography (CCT) and is a robust and well established tool for risk stratification of patients with suspected coronary artery disease (CAD).1 If performed along with a myocardial perfusion scintigraphy tomography (SPECT-CT) it provides complementary information for image interpretation and incremental prognostic value.2 Moreover, in a recent study a very low long-term mortality rate was reported in patients without coronary calcifications (CACS = 0), while higher CACS were associated with higher risk of cardiac events over a 15-year follow-up period.3
In clinical practice, CCT for the assessment of CACS is commonly acquired with a CT tube voltage set at 120 kilovolt peak (kVp), resulting in an effective radiation dose of approximately 0.6 mSv.4 In addition, a subsequent low-dose CT with 80 kVp tube voltage is commonly acquired for attenuation correction (AC) purpose.5
In order to address the requirement for population dose containment, the possibility to use a single CT scan with tube voltage set at 80 kVp both for AC and for the assessment of CACS would be highly warranted, as recently suggested in other studies featuring small patient cohorts.4,6 The preliminary findings of these latter studies need now to be validated in larger patient populations. The aim of the current study was twofold:
Materials and methods
Study design
This single-center retrospective observational study was conducted in accordance with the declaration of Helsinki. A waiver for retrospective analysis of the patient’s data was obtained by Cantonal Ethics Commission. Consecutive patients without prior history of CAD undergoing myocardial perfusion scintigraphy (MPS) as part of standard clinical care were included.
All underwent standard CACS-CCT scanning with 120-kVp tube voltage and an additional CCT scan with 80 kVp, which was also used for attenuation correction (AC).
Imaging protocol
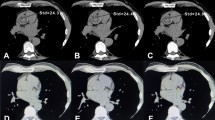
All patients sequentially underwent a standard non-contrast enhanced CT scan (tube voltage 120 kVp, 25 mAs, pitch 1.2, rotation time 2.1 ms, Matrix 128 × 128, collimation 1.6 × 1.2) and a low-dose CT acquisition (tube voltage 80 kVp, 20 mAs, pitch 1.2, rotation time 0.6 ms, Matrix 128 × 128, collimation 1.6 × 1.2). Standard scans were performed in cranio-caudal direction during inspiratory breath-hold with prospective electrocardiogram (ECG)-triggering. Low-dose scans were performed also in cranio-caudal direction, but without breathing instruction and without electrocardiogram (ECG)-triggering. All post-acquisition processing and reconstruction were done in a similar fashion for both the low and standard dose scans. Radiation Dose was approximately 0.6 mSv for the standard protocol and 0.2 to 0.3 mSv for the low-dose protocol.
Image Evaluation
All images were read in consensus by a board-certified nuclear medicine physician and a board-certified cardiologist.
Calcium scoring was done, equally for the 80 and 120 kVp acquisitions using a dedicated semiautomatic software included in Syngo.Via workstations (Siemens Healthineers AG, Erlangen, Germany). In brief, all pixels with an attenuation equal or above the lowest threshold (i.e., ≥ 130 HU) having an area ≥ 1 mm2 are automatically color-marked and then manually selected by creating a region of interest around all lesions found in a coronary artery. The software then calculates the CACS as previously reported,9 by multiplying the density score and the area of calcification.
Based on the CACS derived from both, the 80 and 120-kVp scans, each patient was allocated to the corresponding percentile group as reported in the literature9: < 25%, 25% to 50%, 50% to 75%, 75% to 90%, > 90%.
Statistical analysis
Continuous variables are shown as mean with standard deviation unless otherwise specified, P values less than 0.05 were taken to indicate statistical significance. CACS were compared between scans with different tube voltage using Wilcoxon Test after confirmation of a non-normal distribution by means of Kolmogorov–Smirnov test. Statistical equivalence between 80 and 120-kVp CT scan was also assessed by calculating moment correlation coefficients (R and R2) and values were further analyzed by means of Bland–Altman Analysis.
Fisher Exact Test was used to compare differences in the assigned percentile class between 80 and 120-kVp scans. SPSS Software Version 22.0 for Windows was used.
Results
Patient demographics
The study population comprised 1511 consecutive patients (of whom 793 were male (52.5%), mean age 68 ± 9.1 years), all consecutively included between October 2016 and March 2018. The patients had a high cardio-vascular risk profile (BMI 29 ± 5.4 kg/m2, 35.6% with diabetes mellitus, 69% with arterial hypertension, 20.6% had a family predisposition, and 48.5% had dyslipidemia) as reported in Table 1. Prior to image acquisition all patients underwent adequate stress-testing, either pharmacological or with ergometry.
CAC scoring
With regard to standard CT acquisitions with 120 kVp tube voltage, median CACS was 134 (inter-quartile range 8 to 625). 378 (25.0%) patients were assigned to the < 25% percentile class, of whom 264 (17.5%) had CACS = 0. Patients assigned to the other percentile classes were 259 (17.1%) in the 25% to 50%, 341 (22.6%) in the 50% to 75%, 252 (16.7%) in the 75% to 90%, and 281 (18.6%) in the > 90%, respectively.
Considering low-dose acquisitions with 80 kVp tube voltage, median CACS was 52 (inter-quartile range 0 to 379). 591 (39.1%) patients were assigned to the < 25% percentile class, of whom 437 (29%) had a CACS = 0. Patients assigned to the other percentile classes were 236 (15.5%) in the 25% to 50%, 297 (19.7%) in the 50% to 75%, 200 (13.3%) in the 75% to 90%, and 187 (12.4%) in the > 90%, respectively.
Overall, there was a very good correlation between scores calculated with 120 and 80 kVp tube voltage scans (R = 0.94, R2 = 0.88, P > .001). Bland–Altman limits of agreement of − 563.5 to 871.9 and a bias of − 154.2 (Figures 1, 2).
There was a difference between the proportion of patients assigned in the < 25% percentile class (P = .03) (25.0% in standard CT acquisition vs 39.1% in low-dose CT acquisition). Conversely, the proportion of patients assigned to the percentile classes other than < 25% did not differ between the two acquisition protocols. The results are summarized in Table 2 and in Figure 3.
Moreover, we found a significant difference in the proportion of patients with CACS = 0 between standard acquisition (n = 264, 17.5%) and low-dose scan (n = 437, 28.9%, P < .001). The 437 patients with CACS = 0 with the low-dose scan had CACS ranging between 0 and 372 (median 0, IQR 0 to 4). Among patients with CACS = 0 in the low-dose imaging, 173 had CACS > 0 on the standard acquisition. Of note, these 173 patients are mostly coming from the < 25% risk class as assessed with the standard protocol. In only 9/173 Patients (5%) the standard acquisition showed markedly higher values (i.e., from the 25% to 50% risk class, median 6, IQR 1 to 3).
Among those patients assigned to the < 25% percentile class, the proportion of patients with BMI ≥ 25 and BMI ≥ 30 was higher for the low-dose protocol compared to the standard one (38.2% vs 24.3% and 37.6% vs 22.2%, respectively, both P < .001). The same held true for patients with CACS = 0 (28.7% vs 17.0% and 30.7% vs 17.6%, respectively, both P < .001). Of note, the difference in the proportion of patients assigned to the < 25% percentile class was independent of patient sex.
Discussion
CACS is a useful prognostic tool to accurately estimate long-term risk of asymptomatic or symptomatic patients without prior CAD as well as for initial high-risk patients.10,11 It has been shown that patients without coronary calcifications of any degree have a very low mortality risk compared with the general population, while cardiovascular risk progressively increases with higher CAC scores.12
Therefore, the reliability of CACS results is pivotal in the prognostic assessment of patients with suspected CAD, and to identify those patients with the lowest risk of adverse events. Standard protocols for the assessment of CACS fit this need but cause a non-negligible additional radiation burden. It is therefore incumbent upon physicians performing cardiovascular imaging to reduce the radiation burden associated with CACS as far as is practicable. While the mean radiation burden associated with medical procedures has considerably decreased in recent years, there is still the potential for further reduction. An elegant way to reduce the radiation burden is to modify the parameters of CCT acquisition, provided that diagnostic accuracy can be maintained.13
Some studies demonstrated the accuracy of CACS acquired with low-dose protocols using tube voltage set at 80 to 100 kVp. A first phantom study showed an equivalence in accuracy and reproducibility using tube voltages set at 120 and 80 kVp.14 Hecht et al. demonstrated that using protocols with low-dose lung scanning allows for an excellent agreement of CACS-based risk classification at low and standard doses.15
Conversely, other two studies comparing 120 and 100 kVp showed that low tube voltages lead to a systematic overestimation of CACS.16,17 Consistent with these latest results, different HU thresholds were proposed to assess the CACS (e.g., 147 HU instead of 130 HU16).
In this regard, there is a discrepancy between the results of our study and those reported in the literature. In fact, we found a systematic underestimation of calculated CACS if 80 kVp tube voltage was used. As a consequence, the number of patients assigned to the low (< 25%) percentile class was different between the two protocols, being higher for low-dose acquisitions. Additionally, also the proportion of patients with CACS = 0 was different between the two acquisition protocols. The discrepancy may relate in a difference in the patient sample size, being ours larger by a factor of 10 compared with the previous reports. Another possible explanation is the impaired recognition of smaller calcifications with the low-dose protocol, possibly due to increased noise and consequent loss in spatial resolution. In a recent study, reducing the kVp tube voltage caused an increase in image noise by a factor 1.9 and 2.5 at 80 and 70 kVp, respectively.18 Due to image blurring and the concomitant impact of partial volume effect (PVE),19 it is conceivable that some tiny lesions are missed in low-dose protocols, and this becomes evident when larger patients’ samples are investigated.
It could be argued that increased noise at low dose may also cause overestimation in view of lesions which may be wrongly considered as calcium. Alternatively, this overestimation may come from an apparent increase in surface area of the detected calcifications. However, calcium scoring relies on two parameters: weighted density score given to the highest attenuation value and the area of the calcification. Artifact-related lesions might not present with sufficient density to be included in the automatic calcium scoring. On the other hand, even true calcification with increased apparent surface area may not lead to an overestimation of CACS; if images are blurred, density may appear lower. Even a relatively small difference in HU can cause a difference in the weighted density score, which may not be counterbalanced by the increase in area, especially if the density of detectable calcifications falls below 130HU.
In this regard, our results show that the greatest difference and spread in CACS between the two scores becomes more evident in the progressively higher scores and not in the very lowest percentile groups. This observation seems to rule out increased noise as major determinant of underestimated CACS, as the impact of noise should be more evident for small calcifications, as seen in lower CACS. However, as CACS equals the sum of scores of all calcifications, then higher CACS often results from multiple lesions. As a real calcification can be missed or underestimated if a blurred image causes a spread of its appearance, the same repeated error on multiple calcifications would amplify the bias in the calculation of global CACS with consequent underestimation, which intuitively becomes more evident if a large patient population is investigated.
Also, BMI can substantially affect imaging quality. It was shown that BMI is a major factor to predict image quality in patients undergoing a thorax CT: the higher the BMI, the lower the image resolution.20 This was also the case in our study, wherein the proportion of patients with BMI ≥ 25/30 was higher in those patients with lower CACS using our low-dose protocol. The fact that the calculation of CACS can be affected by high BMI, especially if a low-dose protocol is used, has evident implications in clinical practice and suggests that overweight and obese patients should be scanned with standard protocols in order to obtain a reliable calcium scoring. The impact of sex in this regard seems negligible, as the higher proportion of patients in the < 25% percentile class as well as with CACS = 0 was constant across male (P = .50) and female patients (P = .11).
In addition, the lack of breathing instruction in the low-dose protocol may also explain decreased resolution. In fact, it is well known that the patient’s motion can have a major impact on image quality on CT.21 In this regard, we may hypothesize that the relatively short acquisition time and the patients’ compliance could minimize the effect of the lack of breathing indication on the detectability and the precise assessment of small calcifications, but a definite answer cannot be given and should be investigated in future works. It should be noted that all patients were cooperative and that a low-dose CT during MPS for attenuation correction purpose only lasts for one single breath cycle, thus reducing the risk of major motion-related artifacts.
In our paper, we did not use the recently proposed HU thresholds for 80 kVp tube voltages and our choice deserves a further clarification. Gräni et al. proposed in 2018 adapted thresholds for acquisition with tube voltages down to 70 kVp,6 in view of a tendency in their study toward an overestimation of CACS if lower voltages were deployed. In our study the use of such threshold would have rather amplified the underestimation, with evident impact on diagnostic accuracy. Hence, we believe that the use of the same HU thresholds between the two protocols (i.e., 130 HU) is justified here. While the calculation of CACS may be affected by these drawbacks, the fact that the CACS calculated with the standard acquisition within the same patients differed only in a small proportion of patients and to a small extent renders the overall clinical impact probably minor with respect to the assignment to the established risk classes.
However, the impact on the diagnosis (normal vs abnormal) and on the prognostic value should be further discussed. In fact, if a low-dose CCT is used, significantly more patients are considered as not having any coronary calcifications, and this may lead to an underestimation of the cardiovascular risk in these patients. In our patients’ population, 173 Patients (11.6%) were falsely diagnosed as non-CAD due to CACS = 0 with the low-dose protocol, but > 0 with the standard protocol (median 6, IQR 1 to 3). Of note, all patients with CACS = 0 with the standard protocol also had CACS = 0 with low-dose protocol. A low calcium score is still consistent with a favorable prognosis, with the exception of very young patients, as the majority of events occur in individuals with high CACS percentile classes.8 As such, consistent with data from the literature, CACS percentile classes may constitute an effective screening method to stratify individuals at risk as well, and in this regard, our data showed a very good correlation across percentiles classes (r = 0.857, r2 = 0.74, P < .001). But as a matter of fact, the power of zero22 and its ability to predict a long disease-free survival in patients with suspected CAD22 should be considered reliable only if the CACS is assessed by standard protocols. This latter aspect has an evident impact in clinical practice, wherein patients are normally referred to calcium scoring as primary prevention if asymptomatic. In such patients, a precise diagnosis of CAD is essential to plan an adequate primary prevention. The same does not hold true for a patients’ population similar to that of the present study, wherein patients were referred to an ischemia test in view of their high cardiovascular risk and/or symptoms. While a standard protocol is mandatory if CACS is calculated as standalone modality, the same may not hold true if an ischemia test such as MPS is associated. In this latter setting, the implementation of low-dose protocols for the calculation of CACS may be pursued without relevant loss in prognostic power. Thus, low radiation dose protocols might be used without relevant impairment in accuracy, thus maintaining a reliable risk stratification for medical therapy.
Our study has some limitations. Due to the retrospective nature of the present study, the impact of technical artifacts potentially affecting image quality could not be assessed. It should be noted that standard parameters were used regardless of body size, sex and age and were not standardized between the two protocols. Hence, the impact of single parameters (e.g., mAs and rotation time) on calcium scoring could not be assessed as well. Furthermore, we could not investigate the lowest limit of dose reduction able to yield equivalent diagnostic accuracy. It is conceivable that a further reduction in tube voltage may be pursued while maintaining an adequate diagnostic performance, but this concept should be validated in further studies. We could not assess the prognostic value of our calculated CACS with both protocols as no complete follow-up data were available. Specifically, it still needs to be elucidated, whether the small calcifications which have been misdiagnosed with the low-dose protocol would have an impact on patients’ prognosis or on the ability to predict cardiac events. As such more work is needed to clarify whether the same prognostic value of CACS as calculated on standard protocols also pertains to low-dose protocols, and the incremental value over MPS alone should be elucidated. While this was beyond the scope of the present paper, this is of importance to evaluate the real impact on clinical practice. Finally, as mentioned before, the HU thresholds used in this study were the same for standard and low-dose acquisitions. Whether a different adaptation of thresholds is necessary, for example in the lower ranges, is unknown and needs further studies and clinical validations.
New knowledge gained
We demonstrate in a large patient population that CACS calculated with low-dose CT scan correlates well with CACS assessed with standard protocols, but there is a tendency toward an underestimation which may possibly limit its prognostic value.
Conclusion
In a large consecutive patient population, CACS calculated with low-dose CCT scan and those assessed with standard protocol correlated well regarding numeric CACS and risk classes. However, there was a systematic underestimation of CACS with low-dose protocols, causing a significantly higher proportion of patients without detectable coronary calcifications. This may impact the prognostic value of CACS and the established “power of zero” may no longer be warranted if the calcium score is assessed using CT with low tube voltages.
Abbreviations
- AC:
-
Attenuation correction
- CACS:
-
Coronary artery calcium score
- CAD:
-
Coronary artery disease
- AC:
-
Attenuation correction
- CCT:
-
Cardiac computer tomography
- LAD:
-
Left anterior descending artery
- RCA:
-
Right coronary artery
- LCX:
-
Left circumflex artery
- MPS:
-
Myocardial perfusion SPECT-CT
- SPECT:
-
Single photon emission computed tomography
- SRS/SSS/SDS:
-
Summed rest/stress/difference score
References
Zellweger MJ. Risk stratification in coronary artery disease: A patient-tailored approach over the ischaemic cascade. Swiss Med Wkly 2019;149:w20014.
Chang SM, Nabi F, Xu J, Pratt CM, Mahmarian AC, Frias ME. Value of CACS compared with ETT and myocardial perfusion imaging for predicting long-term cardiac outcome in asymptomatic and symptomatic patients at low risk for coronary disease: Clinical implications in a multimodality imaging world. JACC Cardiovasc Imaging 2015;8:134‐44.
Nakanishi R, Li D, Blaha MJ, Whelton SP, Darabian S, Flores FR, et al. All-cause mortality by age and gender based on coronary artery calcium scores. Eur Heart J Cardiovasc Imaging 2015;17:1305‐14.
Grossmann M, Giannopoulos AA, Bechtiger FA, Messerli M, Schwyzer M, Benz DC, et al. Very-low-dose computed tomography for attenuation correction of cadmium-zinc-telluride single photon emission computed tomography myocardial perfusion imaging. J Nucl Cardiol 2020;27:228‐37.
Bernstine H, Sopov V, Yefremov N, Nidam M, Gabbai M, Sosna J, et al. Comparison of 80 and 120 kVp contrast-enhanced CT for attenuation correction in PET/CT, using quantitative analysis and reporter assessment of PET image quality. Clin Radiol 2014;69:e17-24.
Gräni C, Vontobel J, Benz DC, Bacanovic S, Giannopoulos AA, Messerli M, et al. Very-low-dose coronary artery calcium scoring using novel scoring thresholds for low tube voltage protocols—a pilot study. Eur Heart J Cardiovasc Imaging 2018;19:1362‐71.
Hoff JA, Chomka EV, Krainik AJ, Daviglus M, Rich S, Kondos GT. Age and gender distributions of coronary artery calcium detected by electron beam tomography in 35,246 adults. Am J Cardiol 2001;87:1335‐9.
Raggi P, Callister TQ, Cooil B, He ZX, Lippolis NJ, Russo DJ, et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation 2000;101:850‐5.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using veryfast computed tomography. J Am Coll Cardiol 1990;15:827‐32.
Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336‐45.
Chang SM, Nabi F, Xu J, Pratt CM, Mahmarian AC, Frias ME, et al. Value of CACS compared with ETT and myocardial perfusion imaging for predicting long-term cardiac outcome in asymptomatic and symptomatic patients at low risk for coronary disease: Clinical implications in a multimodality imaging world. JACC Cardiovasc Imaging 2015;8:134‐44.
Nakanishi R, Li D, Blaha MJ, Whelton SP, Darabian S, Flores FR, et al. All-cause mortality by age and gender based on coronary artery calcium scores. Eur Heart J Cardiovasc Imaging 2016;17:1305‐14.
Kim KP, Einstein AJ, de González AB. Coronary artery calcification screening: Estimated radiation dose and cancer risk. Arch Intern Med. 2009;169:1188‐94.
Thomas CK, Mühlenbruch G, Wildberger JE, Hohl C, Das M, Günther RW, et al. Coronary artery calcium scoring with multislice computed tomography: In vitro assessment of a low tube voltage protocol. Invest Radiol 2006;41:668‐73.
Hecht HS, de Siqueira ME, Cham M, Yip R, Narula J, Henschke C, et al. Low- vs standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging. 2015;16:358‐63.
Marwan M, Mettin C, Pflederer T, Seltmann M, Schuhbäck A, Muschiol G, et al. Very low-dose coronary artery calcium scanning with high-pitch spiral acquisition mode: Comparison between 120-kV and 100-kV tube voltage protocols. J Cardiovasc Comput Tomogr. 2013:32-8.
Kim S, Lee CJ, Han K, Lee KH, Lee HJ, Park S. Histogram-derived modified thresholds for coronary artery calcium scoring with lower tube voltage. Sci Rep 2021;11:17450.
Holmquist F, Nyman U, Siemund R, Geijer M, Söderberg M. Impact of iterative reconstructions on image noise and low-contrast object detection in low kVp simulated abdominal CT: A phantom study. Acta Radiol 2016;57:1079‐88.
Freiman M, Nickisch H, Prevrhal S, Schmitt H, Vembar M, Maurovich-Horvat P, et al. Improving CCTA-based lesions’ hemodynamic significance assessment by accounting for partial volume modeling in automatic coronary lumen segmentation. Med Phys 2017;44:1040‐9.
Koc GG, Koc Z, Kaniyev T, Kokangul A. Thorax CT dose reduction based on patient features: Effect of patient characteristics on image quality and effective dose. Health Phys 2019;116:736‐45.
Lucaya J, Piqueras J, García-Peña P, Enríquez G, García-Macías M, Sotil J. Low-dose high-resolution CT of the chest in children and young adults: Dose, cooperation, artifact incidence, and image quality. AJR Am J Roentgenol 2000;175:985‐92.
Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, et al. Prospective multicenter imaging study for evaluation of chest pain. Circulation. 2017;136:1993‐2005.
Acknowledgments
We greatly appreciate the help and assistance of our excellent radiographers.
Disclosures
The authors report no disclosures relevant to the manuscript. Federico Caobelli received academic grant support in the past from Mallinckrodt AG and Tillots AG and speaker honoraria from Siemens Healthineers and Bracco, for matters not related to the current study.
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allio, I.R., Caobelli, F., Popescu, C.E. et al. Low-dose coronary artery calcium scoring compared to the standard protocol. J. Nucl. Cardiol. 30, 1191–1198 (2023). https://doi.org/10.1007/s12350-022-03120-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-022-03120-3