Abstract
Background
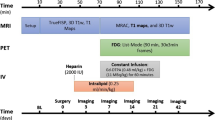
The aims of this study were to investigate the application of a constant infusion (CI) to mitigate the issue of constantly changing Gd-DTPA contrast levels in a bolus injection for extracellular volume (ECV) measurements by (a) comparing a CI alone to a bolus alone and a bolus followed by CI in healthy myocardium, (b) evaluating the impact of glucose suppression using heparin on ECV.
Methods
Five healthy canine subjects were imaged to compare three different protocols for injecting Gd-DTPA and FDG: bolus alone, CI alone, bolus followed by CI. Suppression of myocardial glucose uptake was induced using a continuous infusion of 20% lipid at a rate of 0.25 mL·min−1·kg−1 as well as 2000 units of intravenous heparin injected 20 minutes prior to FDG/Gd-DTPA injection.
Results
There was no significant effect on ECV measurement when heparin was used for glucose suppression at equilibrium irrespective of infusion protocol). Measurements of ECV in myocardium, regardless of infusion protocol showed no significant difference at all time points (P = 0.21) prior to washout.
Conclusions
The suppression of myocardial uptake of [18F]FDG with heparin did not alter the determination of myocardial ECV though a larger sample size may show differences. Further, the infusion protocol (bolus or constant infusion) had no effect on the calculated ECV.







Similar content being viewed by others
Abbreviations
- GBCA:
-
Gadolinium-based contrast agent
- ECV:
-
Extracellular volume
- CI:
-
Constant infusion
- [18F]FDG:
-
18F-fluorodeoxyglucose
- MI:
-
Myocardial infarction
- MBq:
-
Megabecquerel
- PET:
-
Positron emission tomography
- MOLLI:
-
Modified look-locker inversion recovery
References
Kidambi A, Motwani M, Uddin A, Ripley DP, McDiarmid AK, Swoboda PP, et al. Myocardial extracellular volume estimation by CMR predicts functional recovery following acute MI. JACC Cardiovasc Imaging 2017. https://doi.org/10.1016/j.jcmg.2016.06.015.
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 mapping basic techniques and clinical applications. JACC Cardiovasc Imaging 2016. https://doi.org/10.1016/j.jcmg.2015.11.005.
Radenkovic D, Weingärtner S, Ricketts L, Moon JC, Captur G. T1 mapping in cardiac MRI. Heart Fail Rev 2017. https://doi.org/10.1007/s10741-017-9627-2.
Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 2009. https://doi.org/10.1002/jmri.21969.
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000. https://doi.org/10.1056/NEJM200011163432003.
Thornhill RE, Prato FS, Wisenberg G, Moran GR, Sykes J. Determining the extent to which delayed-enhancement images reflect the partition-coefficient of Gd-DTPA in canine studies of reperfused and unreperfused myocardial infarction. Magn Reson Med 2004;52:1069-79. https://doi.org/10.1002/mrm.20236.
Salerno M, Janardhanan R, Jiji RS, Brooks J, Adenaw N, Mehta B, et al. Comparison of methods for determining the partition coefficient of gadolinium in the myocardium using T1 mapping. J Magn Reson Imaging 2013. https://doi.org/10.1002/jmri.23875.
Diesbourg LD, Prato FS, Wisenberg G, Drost DJ, Marshall TP, Carroll SE, et al. Quantification of myocardial blood flow and extracellular volumes using a bolus injection of Gd-DTPA: Kinetic modeling in canine ischemic disease. Magn Reson Med 1992. https://doi.org/10.1002/mrm.1910230205.
Arai AE. The cardiac magnetic resonance (CMR) approach to assessing myocardial viability. J Nucl Cardiol 2011. https://doi.org/10.1007/s12350-011-9441-5.
Wilk B, Wisenberg G, Dharmakumar R, Thiessen JD, Goldhawk DE, Prato FS. Hybrid PET/MR imaging in myocardial inflammation post-myocardial infarction. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01973-9.
Scholtens AM, Verberne HJ, Budde RPJ, Lam MGEH. Additional heparin preadministration improves cardiac glucose metabolism suppression over low-carbohydrate diet alone in 18F-FDG PET imaging. J Nucl Med 2016. https://doi.org/10.2967/jnumed.115.166884.
Prato FS, Butler J, Sykes J, Keenliside L, Blackwood KJ, Thompson RT, et al. Can the inflammatory response be evaluated using 18F-FDG within zones of microvascular obstruction after myocardial infarction? J Nucl Med 2015;56:299–304. https://doi.org/10.2967/jnumed.114.147835.
Joerges J, Schulz T, Wegner J, Schumacher U, Prehm P. Regulation of cell volume by glycosaminoglycans. J Cell Biochem 2012. https://doi.org/10.1002/jcb.23360.
Scholtens AM, van den Berk AM, van der Sluis NL, Esser JP, Lammers GK, de Klerk JMH, et al. Suppression of myocardial glucose metabolism in FDG PET/CT: Impact of dose variation in heparin bolus pre-administration. Eur J Nucl Med Mol Imaging 2020. https://doi.org/10.1007/s00259-020-04713-1.
Lawrence J, Chang YMR, Szladovits B, Davison LJ, Garden OA. Breed-specific hematological phenotypes in the dog: A natural resource for the genetic dissection of hematological parameters in a mammalian species. PLoS ONE 2013. https://doi.org/10.1371/journal.pone.0081288.
White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, et al. T1 mapping for myocardial extracellular volume measurement by CMR. JACC Cardiovasc Imaging 2013. https://doi.org/10.1016/j.jcmg.2013.01.011.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983;3:1–7. https://doi.org/10.1038/jcbfm.1985.87.
Higgins PJ, Garlick RL, Bunn HF. Glycosylated hemoglobin in human and animal red cells. Role of glucose permeability. Diabetes 1982. https://doi.org/10.2337/diab.31.9.743.
Anazodo U, Kewin M, Finger E, Thiessen J, Hadway J, Butler J, et al. Preliminary evaluation of MRI-derived input function for quantitative measurement of glucose metabolism in an integrated PET-MRI. EJNMMI Phys 2015;2:1–2. https://doi.org/10.1186/2197-7364-2-s1-a80.
Lortie M, Beanlands RSB, Yoshinaga K, Klein R, DaSilva JN, DeKemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765–74. https://doi.org/10.1007/s00259-007-0478-2.
Lurz JA, Luecke C, Lang D, Besler C, Rommel KP, Klingel K, et al. CMR-derived extracellular volume fraction as a marker for myocardial fibrosis: The importance of coexisting myocardial inflammation. JACC Cardiovasc Imaging 2018. https://doi.org/10.1016/j.jcmg.2017.01.025.
Haaf P, Garg P, Messroghli DR, Broadbent DA, Greenwood JP, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: A comprehensive review. J Cardiovasc Magn Reson 2016. https://doi.org/10.1186/s12968-016-0308-4.
Thornhill RE, Prato FS, Wisenberg G, White JA, Nowell J, Sauer A. Feasibility of the single-bolus strategy for measuring the partition coefficient of Gd-DTPA in patients with myocardial infarction: Independence of image delay time and maturity of scar. Magn Reson Med 2006. https://doi.org/10.1002/mrm.20830.
Bekkers SCAM, Yazdani SK, Virmani R, Waltenberger J. Microvascular obstruction. Underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol 2010. https://doi.org/10.1016/j.jacc.2009.12.037.
Nensa F, Tezgah E, Schweins K, Goebel J, Heusch P, Nassenstein K, et al. Evaluation of a low-carbohydrate diet-based preparation protocol without fasting for cardiac PET/MR imaging. J Nucl Cardiol 2017. https://doi.org/10.1007/s12350-016-0443-1.
Al-Wakeel-Marquard N, Rastin S, Muench F, Oh-Ici D, Yilmaz S, Berger F, et al. Cardiac T1 mapping in congenital heart disease: Bolus vs. infusion protocols for measurements of myocardial extracellular volume fraction. Int J Cardiovasc Imaging 2017. https://doi.org/10.1007/s10554-017-1191-2.
Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, et al. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: Slow infusion versus bolus. J Cardiovasc Magn Reson 2011. https://doi.org/10.1186/1532-429X-13-16.
Acknowledgments
Wilk, B. is supported by an Ontario Graduate scholarship and a Lawson Internal Research Fund. This work was supported in part by Ontario Research Fund RE7-021 and Canadian Foundation for Innovation No. 11358. The authors would like to thank Siemens Health Care Limited for the in-kind contribution of the Myomaps software licence.
Disclosure
The authors have indicated that they have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smailovic, H., Wilk, B., Wisenberg, G. et al. Simultaneous measurements of myocardial glucose metabolism and extracellular volumes with hybrid PET/MRI using concurrent injections of Gd-DTPA and [18F]FDG. J. Nucl. Cardiol. 29, 1304–1314 (2022). https://doi.org/10.1007/s12350-020-02486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02486-6