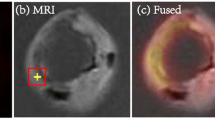
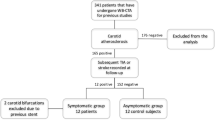
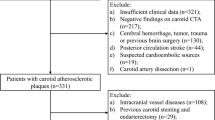
Abstract
Background
Texture analysis has been increasingly used in the field of positron emission tomography (PET)/computed tomography (CT) imaging with Fluorine-18 fluorodeoxyglucose (18F-FDG), aiming at assessing tumor heterogeneity. The purpose of the present study is to examine the feasibility of performing texture analysis in carotid arteries, investigate the value of textural features as predictors of potential plaque vulnerability using as reference standards histological and immunohistochemical data and compare their performance with conventional uptake measurements.
Methods
67 different 18F-FDG PET-based textural features were extracted from carotid images of 21 patients with high-grade carotid stenosis undergoing endarterectomy. To identify the more reliable predictors, univariate logistic regression analysis was performed. The accuracy was satisfactory in case of an Area Under the Receiver Operating Characteristic (ROC) curve (AUC) ≥ 0.80.
Results
First measure of information correlation (AUC = 0.87, P < 0.001), large zone low gray level emphasis (AUC = 0.87, P < 0.001), and normalized run length non-uniformity (AUC = 0.84, P < 0.001) were the most optimal textural features for identifying characteristics of plaque vulnerability based on histological analysis. Addition of textural features to target-to-background ratio (TBR) (AUC = 0.74, P = 0.031) resulted in an AUC = 0.92 (P < 0.001), however, this did not reach statistical significance (Pdiff = 0.09). Intensity histogram standard deviation (AUC = 0.87, P < 0.001) and joint variance (AUC = 0.81, P = 0.001) were the most efficient features for signal differential in relation to immunohistochemical findings and provided incremental value compared to TBR (Pdiff = 0.02).
Conclusion
Texture analysis can be applied in 18F-FDG PET carotid imaging providing valuable information for plaque characterization.





Similar content being viewed by others
Abbreviations
- 18F-FDG:
-
Fluorine-18 fluorodeoxyglucose
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- ROI:
-
Region of interest
- TBR:
-
Target-to-background ratio
- info.correlation1GLCM :
-
First measure of information correlation
- rlnuGLRLM :
-
Run length non-uniformity
- lzlgeGLSZM :
-
Large zone low gray level emphasis
- SDIH :
-
Intensity histogram standard deviation
- varianceGLCM :
-
Joint variance
References
Toutouzas K, Koutagiar I, Benetos G, Aggeli C, Georgakopoulos A, Athanasiadis E, et al. Inflamed human carotid plaques evaluated by PET/CT exhibit increased temperature: Insights from an in vivo study. Eur Heart J Cardiovasc Imaging. 2017;18:1236-44.
Grotta JC. Clinical practice. Carotid stenosis. N Engl J Med. 2013;19:1143-50.
Davies JR, Rudd JH, Weissberg PL. Molecular and metabolic imaging of atherosclerosis. J Nucl Med. 2004;45:1898-907.
Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, et al. Arterial neovascularization and inflammation in vulnerable patients: Early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843-50.
Faggioli GL, Pini R, Mauro R, Pasquinelli G, Fittipaldi S, Freyrie A, et al. Identification of carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography: Correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg. 2011;41:238-48.
Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology. 2014;271:381-9.
Vöö S, Kwee RM, Sluimer JC, Schreuder FH, Wierts R, Bauwens M, et al. Imaging intraplaque inflammation in carotid atherosclerosis with 18F-fluorocholine positron emission tomography-computed tomography: Prospective study on vulnerable atheroma with immunohistochemical validation. Circ Cardiovasc Imaging 2016;9:e004467.
Bucerius J, Hyafil F, Verberne HJ, Slart RH, Lindner O, Sciagra R, et al. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43:780-92.
Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: Carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871-8.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105:2708-11.
Vesey AT, Jenkins WS, Irkle A, Moss A, Sng G, Forsythe RO, et al. 18F-Fluoride and 18F-fluorodeoxyglucose positron emission tomography after transient ischemic attack or minor ischemic stroke: Case-control study. Circ Cardiovasc Imaging 2017;10:e004976.
Derlin T, Tóth Z, Papp L, Wisotzki C, Apostolova I, Habermann CR, et al. Correlation of inflammation assessed by 18F-FDG PET, active mineral deposition assessed by 18F-fluoride PET, and vascular calcification in atherosclerotic plaque: A dual-tracer PET/CT study. J Nucl Med. 2011;52:1020-7.
Paquet N, Albert A, Foidart J, Hustinx R. Within-patient variability of (18)F-FDG: Standardized uptake values in normal tissues. J Nucl Med. 2004;45:784-8.
Kafouris PP, Koutagiar IP, Georgakopoulos AT, Pianou NK, Metaxas MG, Spyrou GM, et al. Adjustment of vascular 2-deoxy-2-[18F]fluoro-D-glucose uptake values over time through a modeling approach. Int J Cardiovasc Imaging. 2019;35:955-64.
Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59:1061-9.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40:133-40.
Burger AI, Vargas HA, Apte A, Beattie BJ, Humm JL, Gonen M, et al. PET quantification with a histogram derived total activity metric: Superior quantitative consistency compared to total lesion glycolysis with absolute or relative SUV thresholds in phantoms and lung cancer patients. Nucl Med Bio. 2014;41:410-8.
Hatt M, Tixier F, Pierce L, Kinahan PE, Le Rest CC, Visvikis D. Characterization of PET/CT images using texture analysis: The past, the present…any future? Eur J Nucl Med Mol Imaging. 2017;44:151-65.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges JP, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52:369-78.
Hatt M, Tixier F, Cheze-le Rest C, Pradier O, Visvikis D. Robustness of intratumour 18F-FDG PET uptake heterogeneity quantification for therapy response prediction in oesophageal carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:1662-71.
El Naqa I, Grigsby P, Apte A, Kidd E, Donnelly E, Khullar D, et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009;42:1162-71.
Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med. 2012;53:693-700.
Groheux D, Majdoub M, Tixier F, Le Rest CC, Martineau A, Merlet P, et al. Do clinical, histological or immunohistochemical primary tumour characteristics translate into different (18)F-FDG PET/CT volumetric and heterogeneity features in stage II/III breast cancer? Eur J Nucl Med Mol Imaging. 2015;42:1682-91.
Saleem BR, Beukinga RJ, Boellaard R, Glaudemans AW, Reijnen MM, Zeebregts CJ, et al. Textural features of 18F-fluorodeoxyglucose positron emission tomography scanning in diagnosing aortic prosthetic graft infection. Eur J Nucl Med Mol Imaging. 2017;44:886-94.
Awad J, Krasinski A, Parraga G, Fenster A. Texture analysis of carotid artery atherosclerosis from three-dimensional ultrasound images. Med Phys. 2010;37:1382-91.
Valdés Hernández MDC, González-Castro V, Chappell FM, Sakka E, Makin S, Armitage PA, et al. Application of Texture Analysis to Study Small Vessel Disease and Blood-Brain Barrier Integrity. Front Neurol. 2017;8:327.
Kotze CW, Rudd JH, Ganeshan B, Menezes LJ, Brookes J, Agu O, et al. CT signal heterogeneity of abdominal aortic aneurysm as a possible predictive biomarker for expansion. Atherosclerosis. 2014;233:510-7.
Zwanenburg A, Leger S, Vallières M, Löck S. Image biomarker standardisation initiative. eprint arXiv:1612.07003v7[cs.CV] (2018).
Steyerberg EW. Clinical prediction models. A practical approach to development, validation, and updating. New York: Springer; 2009. p. 94-5, 195, 260.
Smith GC, Seaman SR, Wood AM, Royston P, White IR. Correcting for optimistic prediction in small data sets. Am J Epidemiol. 2014;180:318-24.
Han K, Song K, Choi BW. How to develop, validate, and compare clinical prediction models involving radiological parameters: Study design and statistical methods. Korean J Radiol. 2016;17:339-50.
Hosmer DW, Lemeshow S. Applied logistic regression, 2nd ed. Chapter 5. New York: Wiley; 2000. p. 160-4.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837-45.
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774-81.
Cal-Gonzalez J, Li X, Heber D, Rausch I, Moore SC, Schäfers K, et al. Partial volume correction for improved PET quantification in 18F-NaF imaging of atherosclerotic plaques. J Nucl Cardiol. 2018;25:1742-56.
Disclosures
Kafouris P, Koutagiar I, Georgakopoulos A, Spyrou G, Visvikis D and Anagnostopoulos C declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Funding
The study was supported by the project “The Greek research infrastructure for personalized medicine (pMED-GR)(MIS 5002802)”, which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund). George Spyrou is funded by the European Commission Research Executive Agency Grant BIORISE (No. 669026), under the Spreading Excellence, Widening Participation, Science with and for Society Framework. Pavlos Kafouris’ doctoral thesis is co-financed by Greece and the European Union (European Social Fund (ESF)) through the Operational Program “Human Resources Development, Education and Lifelong Learning” in the context of the project “Strengthening Human Resources Research Potential via Doctorate Research” (OPS-5003404), implemented by the State Scholarships Foundation (ΙΚΥ).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kafouris, P.P., Koutagiar, I.P., Georgakopoulos, A.T. et al. Fluorine-18 fluorodeoxyglucose positron emission tomography-based textural features for prediction of event prone carotid atherosclerotic plaques. J. Nucl. Cardiol. 28, 1861–1871 (2021). https://doi.org/10.1007/s12350-019-01943-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01943-1