Abstract
Background
Evaluation of resting myocardial computed tomography perfusion (CTP) by coronary CT angiography (CCTA) might serve as a useful addition for determining coronary artery disease. We aimed to evaluate the incremental benefit of resting CTP over coronary stenosis for predicting ischemia using a computational algorithm trained by machine learning methods.
Methods
252 patients underwent CCTA and invasive fractional flow reserve (FFR). CT stenosis was classified as 0%, 1-30%, 31-49%, 50-70%, and >70% maximal stenosis. Significant ischemia was defined as invasive FFR < 0.80. Resting CTP analysis was performed using a gradient boosting classifier for supervised machine learning.
Results
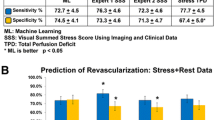
On a per-patient basis, accuracy, sensitivity, specificity, positive predictive, and negative predictive values according to resting CTP when added to CT stenosis (>70%) for predicting ischemia were 68.3%, 52.7%, 84.6%, 78.2%, and 63.0%, respectively. Compared with CT stenosis [area under the receiver operating characteristic curve (AUC): 0.68, 95% confidence interval (CI) 0.62-0.74], the addition of resting CTP appeared to improve discrimination (AUC: 0.75, 95% CI 0.69-0.81, P value .001) and reclassification (net reclassification improvement: 0.52, P value < .001) of ischemia.
Conclusions
The addition of resting CTP analysis acquired from machine learning techniques may improve the predictive utility of significant ischemia over coronary stenosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent advances in computed tomography technology have allowed cardiac computed tomography angiography (CCTA) to become an important non-invasive diagnostic modality for anatomic assessment of the coronary arteries among individuals with suspected coronary artery disease (CAD).1 Although coronary stenosis assessment by CCTA demonstrates high sensitivity and negative predictive values, only about half of the anatomically obstructive lesions detected by CCTA are physiologically significant of ischemia.2 To address this important limitation, much effort has been made by prior studies to combine CCTA with stress imaging.3,4 Despite a combined approach showing increased diagnostic performance for detecting ischemia, these approaches require additional radiation exposure, and are costly and time consuming.
Computed tomography myocardial perfusion (CTP) imaging is an emerging tool that is used for detecting myocardial ischemia.5 Resting CTP can be evaluated by routine clinical CCTA without additional contrast injection or radiation exposure for stress imaging. Although it is well established that the stress phase of perfusion imaging is related to ischemia, prior studies have demonstrated the presence, albeit to a lesser degree, of reduced rest hyperemic blood flow for stenotic lesions. To this end, rest-only myocardial perfusion may also reflect the degree of coronary artery stenosis.6,7 Further still, the high spatial resolution of CT and vasodilatory effect of contrast medium might contribute towards promising results for resting CTP analysis.8,9 Indeed, prior studies reported that resting CTP in CCTA provides additional diagnostic value for predicting obstructive CAD or acute coronary syndromes (ACS).10,11,12
Fractional flow reserve (FFR) is the gold standard for determining physiologically significant CAD. Notably, in the FAME study, an FFR-guided treatment strategy demonstrated significantly improved MACE outcomes compared to an anatomically-guided treatment strategy.13 However, despite the benefits of FFR, its use is limited due to the invasive nature of the procedure. In light of this, the combination of CCTA and resting CTP analysis may prove beneficial for predicting physiological significant ischemia in a non-invasive manner. To date, the role of resting CTP for predicting ischemia by FFR is yet to be firmly established.
In a previous study, our group developed software for resting perfusion analysis using a machine learning technique and validated its diagnostic performance against coronary stenosis detected by invasive angiography.14 In this study, we aimed to test the incremental value of resting perfusion analysis by a machine learning approach over coronary stenosis by CCTA for predicting significant ischemia as determined by FFR.
Methods
Study Population
We studied 252 consecutive adult stable patients with suspected CAD from the DeFACTO (Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography) study, who underwent both clinically indicated non-emergent CCTA and ICA.15 In brief, the DeFACTO study (NCT01233518) is a prospective, multicenter study performed at 17 centers in 5 countries [Canada (n = 1), Belgium (n = 1), Latvia (n = 1), South Korea (n = 2), and United States (n = 12)].16 ICA with intended FFR were performed within 60 days of CCTA and no intervening coronary events were observed. Patients were not deemed eligible for participation if they had a prior history of coronary artery bypass graft surgery, percutaneous coronary intervention with suspected in-stent restenosis based upon CCTA findings, contraindication to adenosine, suspicion of or recent ACS, complex congenital heart disease, pacemaker or defibrillator, prosthetic heart valve, significant arrhythmia, serum creatinine level greater than 1.5 mg/dL, allergy to iodinated contrast, pregnant state, body mass index >35 kg/m2, evidence of active clinical instability or life-threatening disease, or an inability to adhere to study procedures. The appropriate institutional review board committees approved the study protocol and all patients provided written informed consent.
Invasive Coronary Angiography
Selective ICA was performed by standard protocol in accordance with the American College of Cardiology guidelines for coronary angiography,17 with a minimum of 2 projections obtained per-vessel distribution and with angles of projection optimized according to the cardiac position. FFR was performed at the time of ICA (PressureWire Certus, St. Jude Medical Systems, St. Paul, Minnesota; ComboWire, Volcano Corp, San Diego, California) in vessels deemed clinically indicated for evaluation and demonstrating an ICA stenosis between 30% and 90%. After administration of intracoronary nitroglycerin, a pressure-monitoring guide wire was inserted distal to a stenosis. Hyperemia was induced with intravenous administration of adenosine at a rate of 140 μg/kg per minute. FFR was calculated by dividing the mean distal coronary pressure by the mean aortic pressure during hyperemia. In accordance with prior multicenter studies, FFR at a threshold of 0.80 or less was considered significant to indicate lesion-specific ischemia.13
Coronary Computed Tomography Angiography
CCTA was performed with single- or dual-source CT scanners using 64—or higher detector rows with prospective or retrospective electrocardiographic gating in direct accordance with the Society of Cardiovascular Computed Tomography (SCCT) guidelines on performance of CCTA.18,19 An intravenous contrast agent (approximately 80-100 mL), followed by saline (50-80 mL), was injected at a flow rate of 5 mL/s. The scan parameters included heart rate-dependent pitch (0.20-0.45), 330 ms gantry rotation time, 100 or 120 kVp tube voltage, and 350-800 mA tube current. Transaxial images were reconstructed with 0.5- to 0.75-mm slice thickness, 0.3-mm slice increment, 160- to 250-mm field of view, 512 × 512 matrix, and a standard kernel. Optimal phase reconstruction was assessed by comparison of different phases, if available, and the phase with the least amount of coronary artery motion was chosen for analysis. Multiple phases were utilized for image interpretation if minimal coronary artery motion differed among the various arteries. All CTs were interpreted in an intention-to-diagnose approach. CCTA was analyzed using dedicated 3 dimensional workstations (Ziosoft, Redwood City, California; Advantage AW Workstation, GE Healthcare, Milwaukee, Wisconsin) by independent level III experienced readers in a masked fashion. The readers interpreted CCTA images using any or all of the available post-processing image techniques, including 2-dimensional axial and 3-dimensional maximal intensity projection, multiplanar reformat, maximum intensity projections, and short-axis cross-sectional views. In each coronary artery segment, coronary atherosclerosis was defined as tissue structures ≥1 mm2 that existed either within the coronary artery lumen or adjacent to the coronary artery lumen that could be discriminated from surrounding pericardial tissue, epicardial fat, or the vessel lumen itself. Coronary arteries and branches were categorized into 1 of 3 vascular territories: left anterior descending coronary artery (LAD), left circumflex coronary artery (LCX), and right coronary artery (RCA); diagonal branches, obtuse marginal branches, and posterolateral branches were considered part of the left anterior descending coronary artery, LCX, and RCA systems, respectively. The left main coronary artery was considered part of the LAD system. The posterior descending artery was considered part of the RCA or LCX system, depending upon the coronary artery dominance. Stenosis severity was graded in accordance with SCCT guidelines, and categorized as 0%, 1-30%, 31-49%, 50-69%, and ≥70%. High-grade stenosis by CCTA was dichotomized at the 70% threshold, with a stenosis ≥70% considered obstructive. Scan image quality was assessed using a five-point ranking scale as previously described.20
Myocardial Perfusion Analysis
Retrospective image reconstruction was performed during mid-diastole, at 60-75% of the cardiac cycle phase. The images were analyzed with custom software (SmartHeart; Weill Cornell Medicine, New York, USA), which has been previously validated for analyzing perfusion defects using coronary stenosis detected by ICA.14 Briefly, the software employs a compact representation of the left ventricle by subdivision surfaces, which ensures the smoothness of left ventricle geometry with a small number of vertices extracted from CCTA images (Fig. 1A). The thickness of the myocardium is explicitly modeled at each vertex in this representation, enabling the coupling between endocardial and epicardial layers. Using mesh parameterization techniques, the myocardium was divided automatically into an American Heart Association (AHA) 17-segment model (Fig. 1B).21 A total of 51 features were extracted per heart, with three features for each of the 17 segments: normalized perfusion intensity (NPI), transmural perfusion intensity ratio (TPI), and myocardial wall thickness (MWT) were calculated. NPI was computed by first averaging the minimal image intensity on the line segment then dividing by a median of perfusion intensity measurements within a region of interest in the ascending aorta. TPI was defined as the ratio of subendocardium perfusion intensity to subepicardial perfusion intensity in each segment. MWT was computed as the average thickness over all points belonging to each myocardial segment. Further details regarding these three features have been documented in a prior study.14
Development LV segmentation and 17-segment AHA model using SmartHeart software. Reprinted with permission from the Journal of Medial Image Analysis.14 (A) LV model is consisted with the control mesh (vertices in red and edges in black). The midcardial surface (M mid) is modeled as subdivision surface. The endocardial (M endo) and epicardial surfaces (M epi) are represented by warping inwards and outwards along normal directions by half of the thickness each. (B) Mapping from a 3D point × to a 2D point (u,v) within a unit circle. LV, left ventricle; AHA, American Heart Association
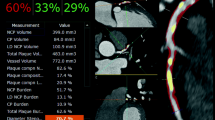
Based on these three features from resting perfusion analysis—NPI, TPI, and MWT—perfusion prediction analysis was performed using a gradient boosting classifier in a supervised machine learning method.22 For the validation strategy, we employed the “leave-one-out” cross-validation method to obtain the likelihood (%) of ischemia on both a per-patient and per-vessel level.23 An example of this analysis is displayed in Fig. 2. For the per-vessel analysis, myocardial segments were assigned to one of the three major coronary artery territories as defined by AHA guidelines.21
An example of perfusion, transmural perfusion ratio, and myocardial wall thickness quantified using SmartHeart software in individuals with non-obstructive coronary stenosis by CCTA causing ischemia. (A) 2nd obtuse marginal branch shows less than 50% stenosis indicating non-obstructive lesion in CCTA. (B) Corresponding invasive angiogram demonstrating approximately 70% stenosis in the same branch of the circumflex coronary artery. The FFR value was 0.71, indicating ischemia. (C) The color maps demonstrate the measurement of perfusion intensity based on the AHA 17-segment model, which include (D) normalized perfusion intensity (NPI) within each segment divided by aorta reference, (E) transmural perfusion intensity ratio (TPI) averaged within each segment, as well as (F) myocardial wall thickness (MWT). Probability for significant ischemia was 0.59 when using machine learning analysis in this case. CCTA, coronary computed tomography; FFR, fractional flow reserve; AHA, American Heart Association
Statistical Methods
Continuous variables are reported as mean ± standard deviation, and categorical variables are reported as counts (percentages). The optimal cut-off value for the likelihood (%) to differentiate ischemic lesion was determined using Liu’s method.24 The relationship between the likelihood of resting CTP and significant ischemia was assessed using linear regression analyses reporting beta coefficients with standard errors (SE). Dominance analysis was employed to determine the relative importance of each feature derived from resting CTP analysis (e.g., NPI, TPI, and MWT) for predicting the likelihood of ischemia.25 We used general dominance analysis with the ensemble method in the current investigation. The general dominance weights of predictors were calculated by aggregating results across all combinations of the independent variables in the prediction model.25,26 General dominance weight was reported as the rank order according to the importance of each individual feature, and a standardized weight that represented the contribution of a variable-to-variance was explained. The diagnostic performance of resting CTP incremental to CT stenosis for predicting physiological significant CAD was calculated using sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) indexes. Area under the receiver operating characteristic curve (AUC) analyses were used to examine the discrimination for each diagnostic imaging method, and AUC values were compared using the method reported by DeLong et al.27 In addition, as a sensitivity analyses, we recalculated AUC after excluding the patients with known CAD (prior MI or prior PCI, n = 24). Category-free net reclassification improvement (NRI) indexes were utilized to determine the correct reclassification according to resting CTP when added to CT stenosis. A P value < .05 was considered statistically significant. Statistical analyses were performed using SAS (version 9. 3; SAS Institute, Cary, NC, USA) and STATA (version 14; StataCorp, College Station, TX, USA).
Results
Study Population
Of 252 patients, mean age was 63 ± 8.7 years and 70.6% (n = 178) were male. Of the study sample, the number of patients who experienced a previous myocardial infarction (n = 15, 6.0%) or previous percutaneous coronary intervention (n = 16, 6.4%) was relatively low (Table 1). Among 252 CT scans, 215 (85.3%) had excellent image quality and 34 (13.5%) had good image quality, while 2 (0.8%) and 1 (0.4%) had fair and poor image quality, respectively.
The Relationship Between NPI, TPI, and MWT in Resting CTP Analysis
In linear regression analysis, each feature was related with the likelihood of having a perfusion defect by resting CTP analysis (Table 2). Dominance analysis of the three features revealed that TPI was the highest ranked parameter among the CTP features, accounting for 60.8% of the predicted variance for the likelihood of having a perfusion defect according to resting CTP analysis.
Relationship Between Resting CTP Analysis and Invasive FFR
In total, 129 patients (51%) and 151 vessels (37%) presented with significant ischemia. Among three resting CTP analysis features, NPI and MWT were correlated with the invasive FFR (P value .001, for both), however, TPI did not show direct relationship with the invasive FFR (P value .248) (Electronic Supplementary Material 1-3). Resting CTP analysis estimated a likelihood of 58 ± 14% of ischemia among those with and 48 ± 17% in those without significant ischemia (P value < .001). The optimally derived cut-off value for likelihood (e.g., 53.3%) of resting CTP yielded a 62.8% sensitivity, 64.2% specificity, 64.8% positive predictive value, and 62.2% negative predictive value (Table 3).
Diagnostic Performance of Resting CTP When Added to CT Stenosis for Predicting Ischemia
On a per-patient basis, the diagnostic accuracy of abnormal resting CTP in combination with CT stenosis (>70% stenosis) for predicting ischemia was 68.2%, indicating somewhat higher diagnostic performance than resting CTP (63.5%) or CT stenosis (65.8%) alone. Although sensitivity and NPV were somewhat higher for CT stenosis alone, notably, the combination of resting CTP with CT stenosis demonstrated substantially higher specificity (85.4%) and PPV (78.2%), as compared with the specificity (61%) and PPV (65.5%) for CT stenosis alone (P value < .001 and .03, respectively). On a per-vessel basis, the diagnostic accuracy of combined resting CTP with CT stenosis was similar compared with CT stenosis alone (e.g., 73.5%, for both). Further, there is no added benefit of resting CTP analysis over CT stenosis for diagnostic performance in the per-vessel basis analysis (Table 4).
Discrimination and Reclassification of Resting CTP When Added to CT Stenosis
On a per-patient basis analysis, compared with CT stenosis alone, combining resting CTP with CT stenosis demonstrated superior discrimination {AUC, 0.68 [95% confidence interval (CI) 0.62-0.74] vs 0.76 [95% CI 0.70-0.82], respectively; difference, 0.092; P < .001} (Table 5; Fig. 3A). Also, in Table 5, the addition of resting CTP to CT stenosis significantly improved reclassification beyond CT stenosis alone (category-free NRI: 0.59; P value < .001). On a vessel basis analysis, however, the addition of resting CTP to CT stenosis did not elicit an improvement in discrimination beyond CT stenosis alone (Fig. 3B).
As a sensitivity analysis, we restricted the sample population to include only patients without prior PCI and MI (n = 228). Combining resting CTP with CT stenosis still displayed higher discrimination when compared with CT stenosis alone [AUC, 0.75 (95% CI 0.69-0.81) vs 0.66 (0.60-0.72), respectively; difference, 0.089; P < .001] (Electronic Supplementary Material 4).
Discussion
In this international multicenter study, resting CTP analysis using a novel machine learning-based algorithm demonstrated a significant and independent association with physiologically significant CAD. Further, resting CTP analysis improved diagnostic performance and discrimination for predicting significant ischemia over anatomical stenosis defined by CCTA alone. Specifically, the addition of resting CTP to CT stenosis substantially augmented diagnostic specificity, with a threefold reduction in patients with a false positive finding. To the best of our knowledge, this is the first study that demonstrates the diagnostic value of resting CT perfusion for predicting physiological significant CAD as defined by invasive FFR, utilizing a novel automated machine learning approach.
Previous studies have shown that stress CTP affords reasonable accuracy for detecting myocardial ischemia,5 however, some concern remains as to whether patients are exposed to additional radiation, contrast, and administration of medication during the administration of stress CTP that could perhaps provoke serious adverse events, particularly in those with significant lesion ischemia. In contrast, resting CTP analysis can be evaluated using routine CCTA images without the need for additional procedures, rendering it a more safer and convenient approach for patient evaluation of ischemia.
There are several reasons that perhaps explain why ischemia can be detected in even in rest-only CTP imaging. Briefly, myocardial blood flow can maintain homeostasis by auto-regulation of hydrostatic pressure despite increasing degrees of coronary stenosis.6 However, if the degree of coronary stenosis rises above a certain level, auto-regulation phenomenon is exhausted and myocardial blood decreased. Reduced myocardial blood flow in patients without myocardial infarction is then observed as decreased attenuation in resting myocardial perfusion imaging.7 In addition, advancements in CT technology permit the assessment of high spatial resolution compared with other perfusion imaging modalities—such as cardiac magnetic resonance imaging or positron emission tomography—and may allow for mild forms of subendocardial ischemia. Branch et al11 reported that resting CTP improved detection of ACS in patients who presented to the emergency department with suggested ACS. In this study, although resting CTP improved the detection of ACS, the results showed limited value for exclusion of ACS. In another study, Yang et al28 reported the discriminatory power of CTP for predicting hemodynamically significant stenosis. The findings of that study indicated that visual analysis of stress CTP provides incremental value over CCTA. Yet, the AUC relative to resting CTP alone for predicting myocardial ischemia was only 0.52. Given the relatively poor signal to noise of myocardial perfusion at lower levels of blood flow, the poor diagnostic inaccuracy of visual CTP is unsurprising.
In the present study, we automated myocardial perfusion analysis on a myocardial segment basis (i.e., hypo-enhancement of myocardium in resting CTP) to improve detection of hypoperfusion. In addition, we further examined myocardial thickness and the ratio of subendocardium perfusion to subepicardial perfusion (e.g., MWT and TPI). The myocardial thickness and ratio of subendocardium perfusion to subepicardial perfusion are easily obtainable parameters when performed using CTP analysis and are closely related to ischemia. Indeed, previous data suggest that a high myocardial thickness is associated with atherosclerotic burden and elevated inflammatory activity,29 and was found to be an independent predictor of adverse events in addition to coronary atherosclerosis and myocardial ischemia.30 It is also well known that there are significant relationships between the subendocardium-to-subepicardial perfusion ratio and ischemia. Particularly, subendocardium is more vulnerable to coronary stenosis because of potential transmural differences in vascular anatomy.31 These approaches are known to predict significant ischemia even on the background of only resting imaging as reported in the current study.
Taking into consideration these three potential approaches (e.g., NPI, TPI and MWT) from resting CTP analysis, we used a machine learning-based method to obtain the probability of significant ischemia. Machine learning analysis is developed based on a pattern recognition and computational learning theory in artificial intelligence, and can identify patterns and relationships formed from complex databases.32 Though widespread in computer science and commonly used in internet-based text searches, more recently its use has been applied to other areas including medical research. To this end, machine learning applications are being utilized for genetics and DNA analysis.33 As an example, machine learning methods have been employed for the purpose of clinical tasks such as the prediction of cancer prognosis.34 In this study, this analytic approach was applied to calculate the likelihood proportion of having significant ischemia. A major finding was that when added to CCTA, resting CTP analysis significantly improved specificity and PPV as compared with CCTA alone, and also provided incremental benefit over CT stenosis for predicting significant ischemia. Despite our significant findings, the clinical usefulness of resting CTP analysis is still unclear due to loss of sensitivity and NPV when resting CTP analysis is added to CT stenosis alone. In addition, the improvement of discrimination is somewhat marginal (delta AUC: 0.09). Therefore, future studies are warranted to evaluate the utility of resting CTP analysis for lesions with significant ischemia.
Our study has several potential limitations that should be emphasized. Our study population comprised of predominantly Caucasian males with a high prevalence of risk factors, who were indicated to undergo invasive angiography. Therefore, caution should be taken when attempting to extrapolate these findings to the general population as a whole. Invasive FFR was performed only in those vessels that were clinically indicated for safety when performing FFR in non-clinically indicated coronary vessel. Therefore, we cannot discount the possibility that determining the presence of atherosclerosis in other unmeasured coronary vessels could have influenced the presence of ischemia observed in this study. A current drawback of the SmartHeart program is its inability to assess: (1) the anatomic variability in the coronary artery blood supply to myocardial segment, and (2) the myocardial segment that supplies coronary artery blood according to the location of the stenosis in each coronary artery. Further, it is likely that the affected myocardial area by coronary artery stenosis will significantly differ according to the anatomical variability or location of the stenosis. Consequently, this might have influenced the non-significant results according to the per-vessel analysis in the current study. Lastly, although we used the “leave one out” cross-validation method for our validation processing of the machine learning algorithm, further validation testing and a validation cohort might be needed to test the performance of the resting CTP analysis algorithm more definitively.
New Knowledge Gained
Machine learning-based prediction algorithms may identify ischemia with resting perfusion images in routine CCTA without additional procedures.
Conclusions
In conclusion, the use of this novel machine learning-based analysis resulted in improved diagnosis and reclassification of individuals with FFR-based ischemia albeit its present clinical utilization is still unclear. These findings demonstrate the potential feasibility of resting CTP for identifying patients with physiologically significant ischemia without the need for additional stress imaging testing or other invasive procedures.
Abbreviations
- CCTA:
-
Cardiac computed tomography angiography
- CAD:
-
Coronary artery disease
- CTP:
-
Computed tomography myocardial perfusion
- ACS:
-
Acute coronary syndromes
- FFR:
-
Fractional flow reserve
- NPI:
-
Normalized perfusion intensity
- TPI:
-
Transmural perfusion intensity ratio
- MWT:
-
Myocardial wall thickness
- AUC:
-
Area under the receiver operating characteristic curve
- NRI:
-
Category-free net reclassification improvement
References
Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324-36.
Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses: Computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol 2008;52:636-43.
Di Carli MF, Dorbala S, Curillova Z, Kwong RJ, Goldhaber SZ, Rybicki FJ, et al. Relationship between CT coronary angiography and stress perfusion imaging in patients with suspected ischemic heart disease assessed by integrated PET-CT imaging. J Nucl Cardiol 2007;14:799-809.
Gaemperli O, Schepis T, Valenta I, Husmann L, Scheffel H, Duerst V, et al. Cardiac image fusion from stand-alone SPECT and CT: Clinical experience. J Nucl Med 2007;48:696-703.
Ko BS, Cameron JD, Meredith IT, Leung M, Antonis PR, Nasis A, et al. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: A comparison with fractional flow reserve. Eur Heart J 2012;33:67-77.
Jayaweera AR, Wei K, Coggins M, Bin JP, Goodman C, Kaul S. Role of capillaries in determining CBF reserve: New insights using myocardial contrast echocardiography. Am J Physiol 1999;277:H2363-72.
Lindner JR, Skyba DM, Goodman NC, Jayaweera AR, Kaul S. Changes in myocardial blood volume with graded coronary stenosis. Am J Physiol 1997;272:H567-75.
Nikolaou K, Knez A, Rist C, Wintersperger BJ, Leber A, Johnson T, et al. Accuracy of 64-MDCT in the diagnosis of ischemic heart disease. AJR Am J Roentgenol 2006;187:111-7.
Baile EM, Pare PD, D’Yachkova Y, Carere RG. Effect of contrast media on coronary vascular resistance: Contrast-induced coronary vasodilation. Chest 1999;116:1039-45.
Osawa K, Miyoshi T, Koyama Y, Hashimoto K, Sato S, Nakamura K, et al. Additional diagnostic value of first-pass myocardial perfusion imaging without stress when combined with 64-row detector coronary CT angiography in patients with coronary artery disease. Heart 2014;100:1008-15.
Branch KR, Busey J, Mitsumori LM, Strote J, Caldwell JH, Busch JH, et al. Diagnostic performance of resting CT myocardial perfusion in patients with possible acute coronary syndrome. AJR Am J Roentgenol 2013;200:W450-7.
Pursnani A, Lee AM, Mayrhofer T, Ahmed W, Uthamalingam S, Ferencik M, et al. Early resting myocardial computed tomography perfusion for the detection of acute coronary syndrome in patients with coronary artery disease. Circ Cardiovasc Imaging 2015;8:e002404.
Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213-24.
Xiong G, Kola D, Heo R, Elmore K, Cho I, Min JK. Myocardial perfusion analysis in cardiac computed tomography angiographic images at rest. Med Image Anal 2015;24:77-89.
Min JK, Berman DS, Budoff MJ, Jaffer FA, Leipsic J, Leon MB, et al. Rationale and design of the DeFACTO (determination of fractional flow reserve by anatomic computed tomographic AngiOgraphy) study. J Cardiovasc Comput Tomogr 2011;5:301-9.
Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237-45.
Scanlon PJ, Faxon DP, Audet AM, Carabello B, Dehmer GJ, Eagle KA, et al. ACC/AHA guidelines for coronary angiography. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Coronary Angiography). Developed in collaboration with the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 1999;33:1756-824.
Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, et al. SCCT guidelines for performance of coronary computed tomographic angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr 2009;3:190-204.
Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, et al. SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr 2011;5:198-224.
Shim SS, Kim Y, Lim SM. Improvement of image quality with beta-blocker premedication on ECG-gated 16-MDCT coronary angiography. AJR Am J Roentgenol 2005;184:649-54.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42.
Friedman JH. Greedy function approximation: A gradient boosting machine. Ann Stat 2001;29:1189-232.
Zhang T. A leave-one-out cross validation bound for kernel methods with applications in learning. Lect Notes Artif Intell 2001;2111:427-43.
Liu X. Classification accuracy and cut point selection. Stat Med 2012;31:2676-86.
Azen R, Traxel N. Using dominance analysis to determine predictor importance in logistic regression. J Educ Behav Stat 2009;34:319-47.
Gromping U. Estimators of relative importance in linear regression based on variance decomposition. Am Stat 2007;61:139-47.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988;44:837-45.
Yang DH, Kim YH, Roh JH, Kang JW, Han D, Jung J, et al. Stress myocardial perfusion CT in patients suspected of having coronary artery disease: Visual and quantitative analysis-validation by using fractional flow reserve. Radiology 2015;276:715-23.
Mehta SK, Rame JE, Khera A, Murphy SA, Canham RM, Peshock RM, et al. Left ventricular hypertrophy, subclinical atherosclerosis, and inflammation. Hypertension 2007;49:1385-91.
Roever L, Pinto IM, Chagas AC. The association of left ventricular mass with coronary atherosclerosis and myocardial ischemia: Cause and effect or simple association? Eur Heart J Cardiovasc Imaging 2015;16:156-7.
Algranati D, Kassab GS, Lanir Y. Why is the subendocardium more vulnerable to ischemia? A new paradigm. Am J Physiol Heart Circ Physiol 2011;300:H1090-100.
Provost F, Kohavi R. Guest editors’ introduction: On applied research in machine learning. Mach Learn 1998;30:127-32.
Libbrecht MW, Noble WS. Machine learning applications in genetics and genomics. Nat Rev Genet 2015;16:321-32.
Kourou K, Exarchos TP, Exarchos KP, Karamouzis MV, Fotiadis DI. Machine learning applications in cancer prognosis and prediction. Comput Struct Biotechnol J 2015;13:8-17.
Funding
This study was supported by National Institute of Health under Grant #R21HL132277 and a generous gift from the Dalio Institute of Cardiovascular Imaging (New York, NY, USA) and the Michael Wolk Foundation (New York, NY, USA).
Disclosure
Dr. James Min serves as a consultant to HeartFlow. All other authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Donghee Han and Ji Hyun Lee have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Scatter plot for the relationship between each component of resting CTP analysis and invasive fractional flow reserve value. 1. Normalized perfusion intensity (NPI) (TIFF 1521 kb)
Supplementary Fig. 2
Scatter plot for the relationship between each component of resting CTP analysis and invasive fractional flow reserve value. 2. Transmural perfusion intensity (TPI) (TIFF 1521 kb)
Supplementary Fig. 3
Scatter plot for the relationship between each component of resting CTP analysis and invasive fractional flow reserve value. 3. Myocardial wall thickness (NWT) (TIFF 1521 kb)
Supplementary Fig. 4
Receiver operating characteristic curves of per-patient performance of CT stenosis, resting CTP and resting CTP added to CT stenosis for detecting significant ischemia in patients without prior history of myocardial infarction or revascularization (n = 228). CT, computed tomography; CTP, computed tomography perfusion (TIFF 1521 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Han, D., Lee, J.H., Rizvi, A. et al. Incremental role of resting myocardial computed tomography perfusion for predicting physiologically significant coronary artery disease: A machine learning approach. J. Nucl. Cardiol. 25, 223–233 (2018). https://doi.org/10.1007/s12350-017-0834-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-017-0834-y