Abstract
Objective
To determine if increased epicardial adipose tissue (EAT) measured by cardiac CT could be associated with impaired myocardial flow reserve (MFR) in patients with non-obstructive coronary artery disease (CAD).
Background
Studies have shown that EAT volume is related to epicardial obstructive CAD, myocardial ischemia and major adverse cardiac events. However, the association between EAT with coronary microvascular dysfunction and impaired MFR has not been well clarified.
Methods
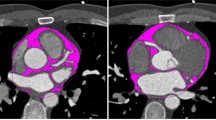
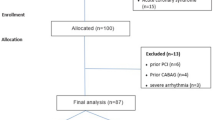
Consecutive patients who underwent Rb-82 positron emission tomography (PET), coronary artery calcium (CAC) scoring and non-invasive coronary computed tomography angiography (CCTA) were screened. PET scans were analysed for standard myocardial perfusion (MPI) and MFR. CCTA results were analysed and only patients with non-obstructive CAD (<50% luminal diameter stenosis) were included. EAT thickness and volumes were measured from CT scans.
Results
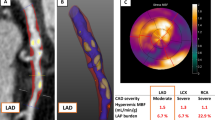
Of 137 patients without obstructive CAD by CCTA and with normal Rb-82 PET relative MPI, 26 (19.0%) patients had impaired MFR < 2 and 87 (64%) patients had CAC. EATthickness, EATvolume and CAC values were higher in patients with impaired MFR < 2 than those with normal MFR ≥ 2 (6.7 ± 1.6 mm vs 4.4 ± 1.0 mm, P < .0001; 119.0 ± 25.3 cm3 vs 105.8 ± 30.5 cm3, P < .04 and 508.9 ± 554.3 vs 167.8 ± 253.9, P < .0001, respectively). However, EATthickness had a stronger negative correlation with MFR than EATvolume and CAC (r = −0.78 vs r = −0.25 and ρ = −0.32, P < .0001). With multivariable logistic regression analysis, only EATthickness was independently associated with impaired MFR (OR 20.7, 95% CI 4.9-87.9, P < .0001). Importantly, the receiver-operator characteristic (ROC) curves demonstrated a superior performance of EATthickness vs EATvolume and EATthickness vs CAC in detecting impaired MFR (AUC: 0.945 vs 0.625, difference between AUC: 0.319, P < .0001; AUC: 0.945 vs 0.710, difference between AUC: 0.235, P < .0006, respectively). On ROC curve analysis, an EATthickness cut-off value > 5.6 mm was optimal in detecting impaired MFR with a sensitivity and specificity of 81% and 92%, respectively.
Conclusions
Increased EAT appears to be associated with impaired MFR. This parameter may help improve detection of patients at risk of microvascular dysfunction.





Similar content being viewed by others
References
Greif M, Becker A, von Ziegler F, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2009;29:781-6.
Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol 2012;110:534-8.
Bucci M, Joutsiniemi E, Saraste A, et al. Intrapericardial, but not extrapericardial, fat is an independent predictor of impaired hyperemic coronary perfusion in coronary artery disease. Arterioscler Thromb Vasc Biol 2011;31:211-8.
Nakazato R, Dey D, Cheng VY, et al. Epicardial fat volume and concurrent presence of both myocardial ischemia and obstructive coronary artery disease. Atherosclerosis 2012;221:422-6.
Janik M, Hartlage G, Alexopoulos N, et al. Epicardial adipose tissue volume and coronary artery calcium to predict myocardial ischemia on positron emission tomography-computed tomography studies. J Nucl Cardiol 2010;17:841-7.
Tamarappoo B, Dey D, Shmilovich H, et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging 2010;3:1104-12.
Cheng VY, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging 2010;3:352-60.
Yerramasu A, Dey D, Venuraju S, et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012;220:223-30.
Schenker MP, Dorbala S, Hong EC, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: A combined positron emission tomography/computed tomography study. Circulation 2008;117:1693-700.
Curillova Z, Yaman BF, Dorbala S, et al. Quantitative relation between coronary calcium content and coronary flow reserve as assessed by integrated PET-CT imaging. Eur J Nucl Med Mol Imaging 2009;36:1603-10.
Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification. J Am Coll Cardiol 2007;49:1860-70.
Iacobellis G, Corradi D, Sharma MA. Epicardial adipose tissue: Anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med 2005;2:536-43.
Baker AR, da Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 2006;5:1.
Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res 2008;40:442-5.
Konishi M, Sugiyama S, Sato Y, et al. Pericardial fat inflammation correlates with coronary artery disease. Atherosclerosis 2010;213:649-55.
Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460-6.
Chatterjee TK, Stoll LL, Denning GM, et al. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circ Res 2009;104:541-9.
Payne GA, Borbouse L, Bratz IN, et al. Endogenous adipose-derived factors diminish coronary endothelial function via inhibition of nitric oxide synthase. Microcirculation 2008;15:417-26.
Eiras S, Teijeira-Fernández E, Shamagian LG, et al. Extension of coronary artery disease is associated with increased IL-6 and decreased adiponectin gene expression in epicardial adipose tissue. Cytokine 2008;43:174-80.
Iacobellis G, Ribando MC, Assal F, et al. Epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endocrinol Metab 2003;88:5163-8.
Corradi D, Maestri R, Callegari S, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol 2004;13:313-6.
McCance KL, Huether SE. Chambers of the heart. Pathophysiology. The biologic basis for disease in adults and children. 4th ed. St. Louis, MO: Mosby; 2002. p. 932.
Ziadi M, de Kemp R, Williams K, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol 2012;19:670-80.
Dilsizian V, Bacharach SL, Beanlands RSB, et al. Imaging guidelines for nuclear cardiology procedures: PET myocardial perfusion and metabolism clinical imaging. J Nucl Cardiol 2009;16:651.
Klein R, Adler A, Beanlands RS, de Kemp RA. Precision-controlled elution of a 82Sr/82Rb generator for cardiac perfusion imaging with positron emission tomography. Phys Med Biol 2007;52:659-73.
de Kemp RA, Nahmias C. Automated determination of the left ventricular long axis in cardiac positron tomography. Physiol Meas 1996;17:95-108.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A Statement for Healthcare Professionals From the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539-42.
Lortie M, Beanlands RS, Yoshinaga K, Klein R, DaSilva JN, de Kemp RA. Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34:1765-74.
Parkash R, Dekemp RA, Ruddy TD, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol 2004;11:440-9.
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830-40.
Chow BJW, Abraham A, Wells GA, et al. Diagnostic accuracy and impact of computed tomographic coronary angiography on utilization of invasive coronary angiography. Circ Cardiovasc Imaging 2009;2:16-23.
Chow BJW, Wells GA, Chen L, et al. Prognostic value of 64-slice cardiac computed tomography: Severity of coronary artery disease, coronary atherosclerosis and left ventricular ejection fraction. J Am Coll Cardiol 2010;55:1017-28.
Abunassar JG, Yam Y, Chen L, D’Mello N, Chow BJ. Usefulness of the Agatston score = 0 to exclude ischemic cardiomyopathy in patients with heart failure. Am J Cardiol 2011;107:428-32.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32.
DeLong ER, Delong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988;44:837-45.
Uren NG, Melin JA, de Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med 1994;330:1782-8.
Graf S, Khorsand A, Gwechenberger M. Typical chest pain and normal coronary angiogram: Cardiac risk factor analysis versus PET for detection of microvascular disease. J Nucl Med 2007;48:175-81.
Reis SE, Holubkov R, Smith AJC, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am Heart J 2001;141:735-41.
Proudfoot D, Shanahan CM. Biology of calcification in vascular cells: Intima versus media. Herz 2001;26:245-51.
Gossl M, Modder UI, Gulati R, et al. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J 2010;31:2909-14.
Huang PH, Chen LC, Leu HB, et al. Enhanced coronary calcification determined by electron beam CT is strongly related to endothelial dysfunction in patients with suspected coronary artery disease. Chest 2005;128:810-5.
Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation 1999;100:1951-7.
Bhagat K, Vallance P. Inflammatory cytokines impair endothelium-dependent dilatation in human veins in vivo. Circulation 1997;96:3042-7.
Wang P, Ba ZF, Chaudry IH. Administration of tumor necrosis factor-alpha in vivo depresses endothelium-dependent relaxation. Am J Physiol 1994;266:H2535-41.
Bhagat K, Collier J, Vallance P. Vasodilatation to arachidonic acid in humans. An insight into endogenous prostanoids and effects of aspirin. Circulation 1995;92:2113-8.
Yoshizumi M, Perrella MA, Burnett JC Jr, et al. Tumor necrosis factor downregulates an endothelial nitric oxide synthase mRNA by shortening its half-life. Circ Res 1993;73:205-9.
Xie J, Wang Y, Lippton H, et al. Tumor necrosis factor inhibits stimulated but not basal release of nitric oxide. Am Rev Respir Dis 1993;148:627-36.
Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150-6.
Conflict of interest
Drs Robert de Kemp, Rob Beanlands and Benjamin Chow received research support from GE Healthcare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, M.S., Green, R., de Kemp, R. et al. Epicardial adipose tissue thickness as a predictor of impaired microvascular function in patients with non-obstructive coronary artery disease. J. Nucl. Cardiol. 20, 804–812 (2013). https://doi.org/10.1007/s12350-013-9739-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-013-9739-6