Abstract
Little is known about acute interstitial pneumonia (AIP) induced by sorafenib therapy in patients with advanced hepatocellular carcinoma (HCC). Here, we present three patients with advanced HCC who developed AIP during sorafenib therapy, with fatal complications in two cases. Case 1 was a 76-year-old man who developed dyspnea. Chest CT showed interstitial pneumonia. Sorafenib was discontinued immediately, and prednisolone was started. His pneumonia resolved. A drug-induced lymphocyte stimulation test for sorafenib was positive. Case 2 was a 75-year-old man and case 3 was a 77-year-old man, both of whom developed high-grade fever and hypoxemia during sorafenib therapy, and were diagnosed with AIP. In spite of high-dose steroid therapy, their respiratory failure worsened and both patients died. In all three cases, serum KL-6 or surfactant protein D concentrations were elevated, and blood and sputum cultures did not grow pathogens. All three patients were smokers with restrictive lung disease on preoperative respiratory function testing, but did not have respiratory symptoms before sorafenib therapy. The clinical features of these three cases suggest that male gender, older age, smoking history, and lung disease are associated with acute sorafenib-induced AIP in patients with advanced HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As systemic chemotherapy has had disappointing results in patients with hepatocellular carcinoma (HCC) [1–5], HCC has generally been considered to be chemoresistant. Sorafenib, a multi-kinase inhibitor that blocks tumor growth and cell proliferation, was the first systemic chemotherapeutic agent found to improve the survival time of patients with advanced HCC in the SHARP trial and Asian Pacific trials [1, 2, 6]. Sorafenib is therefore a novel treatment for patients with advanced HCC. However, it is associated with a low tumor response rate, minimal survival advantage, and high rate of adverse events [1–4].
The reported significant adverse events caused by sorafenib include diarrhea, skin rash (including hand-foot skin reactions), fatigue, liver dysfunction, and hypertension [1–4]. However, there have been few reported cases of interstitial lung disease such as acute interstitial pneumonia (AIP) [7, 8]. Safety information for sorafenib therapy in patients with renal cell carcinoma (RCC) was presented in Japan in December 2008, and reported four cases of acute respiratory failure among 2,000 patients with RCC who had been treated with sorafenib [9]. The clinical features, risk factors, and prognostic factors of sorafenib-induced AIP are not well known at present [7, 8]. As AIP is a life-threatening condition, a better understanding of sorafenib-induced AIP is required. We report three cases of AIP during sorafenib therapy in patients with advanced HCC.
Case reports
Case 1
Case 1 was a 76-year-old man with lymph node metastases from HCC. Serum hepatitis B virus surface antigen and hepatitis C virus (HCV) antibody were negative. He had been given medication for diabetes and a previous myocardial infarction. He was treated with radiofrequency ablation of a single 2.5 cm diameter HCC located in segment 8 of the liver, and had a favorable tumor response. Ten months later, dynamic computed tomography (CT) detected a solitary 2-cm lymph node metastasis at the hepatic hilum. He was asymptomatic with a performance status of 0. Physical examination showed no abnormalities. Laboratory testing showed a decreased serum albumin level of 3.1 g/dL (normal range, >3.7 g/dL), decreased cholinesterase concentration of 197 IU/L (normal range, >220 IU/L), and normal aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels. Liver function was Child–Pugh class A. Serum α-fetoprotein (AFP) level was 2.3 ng/mL (normal range, <10 ng/mL) and serum des-γ-carboxy prothrombin (DCP) level was 2089 mAU/mL (normal range, <39 mAU/mL). Chest X-rays and CT were normal (Fig. 1). Respiratory function testing showed percent predicted vital capacity of 47 %, and he was therefore ineligible for surgical resection of the solitary lymph node metastasis. We treated him with sorafenib 400 mg once daily on an inpatient basis after obtaining written informed consent.
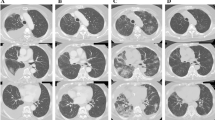
Clinical course of case 1. a Thoracic CT scan findings before sorafenib treatment. No significant findings are present. b Thoracic CT scan findings on the 6th day of sorafenib treatment. Ground-glass opacities (GGOs) are present in the left lung, suggesting interstitial pneumonia. c Thoracic CT scan 12 days after the administration of steroid treatment showing that the GGOs have improved
On the fifth day of sorafenib treatment, he developed progressive dyspnea and fever. Physical examination showed a normal blood pressure of 130/80 mmHg, respiratory rate of 22 breaths/min, pulse rate of 120 beats/min, and body temperature of 37.5 °C. Fine inspiratory crackles were audible over the right lower lung field. Pulse oximetry at rest on room air showed oxygen saturation of 93 %. Arterial blood gas analysis on room air showed a PaO2 of 64 mmHg, PaCO2 of 27 mmHg, and pH of 7.43. Laboratory testing showed an increased lactate dehydrogenase (LDH) concentration of 321 IU/L (normal range, <260 IU/L) and increased C-reactive protein (CRP) concentration of 8.6 mg/dL (normal range, <0.5 mg/dL). Liver and renal function test results were within normal limits. Chest X-rays showed diffuse ground-glass opacities (GGOs) in both lungs. Chest CT showed diffuse interstitial lung disease (Fig. 1). We suspected sorafenib-induced AIP and therefore discontinued sorafenib therapy. No pathogens were cultured from the blood, urine, or sputum. The serum KL-6 concentration was increased at 518 U/mL (normal range, <500 U/mL). A positive drug-induced lymphocyte stimulation test (DLST) for sorafenib confirmed the diagnosis of sorafenib-induced AIP. We initiated prednisolone (PSL) therapy (20 mg/day) on the day we discontinued sorafenib therapy. His symptoms and radiological findings rapidly improved over the following 5 days, and his AIP resolved completely over the following 2 weeks (Fig. 1). He was discharged 16 days after commencing PSL therapy.
Case 2
Case 2 was a 75-year-old male with a past history of pneumothorax. After hepatectomy for HCC, he developed HCV-related multinodular recurrence, and underwent four sessions of transcatheter arterial chemoembolization (TACE). After TACE, dynamic CT showed multiple nodules of recurrent HCC in his remnant liver and we administered sorafenib on an inpatient basis after obtaining written informed consent. Before administration of sorafenib, he was asymptomatic and his vital signs were within normal limits. Physical examination showed no abnormalities. He was not taking any medications. Laboratory testing showed a decreased serum albumin level of 3.4 g/dL, slightly elevated AST level of 45 IU/L, elevated AFP level of 38.5 ng/mL, and normal DCP level of 16 mAU/mL. Liver function was Child–Pugh class A. Chest X-rays and CT were normal (Fig. 2). On the second day of sorafenib therapy, he developed a low-grade fever. His symptoms progressed, and on the 11th day of sorafenib therapy he had high-grade fever and hypoxemia. His oxygen saturation at rest on room air was 92 %. We immediately discontinued sorafenib therapy. Two days later, chest X-rays showed GGOs in both lungs and chest CT showed interstitial lung disease. Laboratory testing showed increased serum concentrations of KL-6 of 1470 U/mL, surfactant protein D (SP-D) of 388 ng/mL (normal range, <109.9 ng/mL), creatine kinase of 1,006 IU/L (normal range, 40–200 IU/L), LDH of 553 IU/L, CRP of 6.6 mg/dL, and immunoglobulin E of 4,150 IU/mL (normal range, <177 IU/mL). No pathogens were cultured from blood or sputum samples.
Clinical course of case 2. a Thoracic CT scan before sorafenib treatment. No significant findings are present. b Thoracic CT scan findings at 13 days of sorafenib treatment. GGOs are present in both lobes, suggesting interstitial pneumonia. c Bronchoscopy findings at 14 days after the onset of sorafenib therapy. The bronchial mucosa was not inflamed and we detected no pus
To establish a definitive diagnosis, we performed bronchoscopy at 14 days after the onset of sorafenib therapy. The bronchial mucosa was not inflamed and we detected no pus. Bronchoalveolar lavage (BAL) showed an increased percentage of lymphocytes (43.0 %) and increased LDH concentration. No pathogens were cultured from BAL fluid samples. We diagnosed AIP, and started steroid pulse therapy with hydrocortisone (1,000 mg/day for 3 days) on the day of bronchoscopy, followed by PSL therapy (60 mg/day). His respiratory function gradually worsened (Fig. 2) and he died of respiratory failure 10 days after developing AIP.
Case 3
Case 3 was a 77-year-old man with HCV-related HCC and a huge sacral bony metastasis. He had previously been treated for hypertension and prostatic hypertrophy. He was treated with TACE for a ruptured HCC, followed by hepatectomy, a further three sessions of TACE, and palliative radiation therapy for the sacral metastasis. Although his performance status was 3 due to paralysis and pain from the bony metastasis, he wished to receive chemotherapy. Physical examination showed no abnormalities other than weakness of the legs. Blood testing showed elevated levels of AST of 58 IU/L, ALT of 63 IU/L, and ALP of 370 IU/L. Liver function was Child–Pugh class A. Chest X-rays and CT were normal (Fig. 3). We administered sorafenib 400 mg twice daily on an inpatient basis after obtaining written informed consent. He tolerated this therapy for over 4 weeks, during which time he developed grade 1 hepatic dysfunction, grade 1 hypertension, and grade 2 hand-foot skin reaction.
On the 41st day of sorafenib treatment, he developed general fatigue and high-grade fever, and we discontinued sorafenib. Two days later, he developed acute dyspnea. Chest X-rays showed diffuse GGOs in both lungs, and chest CT confirmed GGOs with septal thickening throughout both lungs. Laboratory testing showed an increased SP-D concentration of 999 ng/mL. We could not rule out the possibility of a fungal infection because his serum β-D glucan concentration was increased at 56.0 pg/mL (normal range, <20.0 pg/mL). No pathogens were cultured from the blood, urine, or sputum. We suspected sorafenib-associated AIP. Three days after discontinuing sorafenib therapy, we started hydrocortisone (1000 mg/day for 3 days) followed by PSL (30 mg/day), together with antibiotics and oxygen therapy. His respiratory status worsened and he died on the 10th day after developing dyspnea.
Discussion
Commonly reported adverse events caused by sorafenib include diarrhea, skin rash (including hand-foot skin reactions), fatigue, liver dysfunction, and hypertension. However, there have been few reported cases of sorafenib-induced interstitial lung disease [1, 2, 4, 7, 8].
From June 2009 to February 2012, we treated 105 patients with advanced HCC with sorafenib. Six of these patients (5.8 %) developed pulmonary disorders during sorafenib therapy, of which three (2.9 %) were diagnosed with probable sorafenib-induced AIP. The other three patients (2.9 %) with pulmonary disorders had acute bacterial pneumonia and/or carcinomatous lymphangitis. This was a higher incidence of AIP than reported in the all-patient post-marketing surveillance in Japan [10]. The reasons for this high incidence may include the high proportions of smokers and patients with pretreatment respiratory dysfunction in our treatment group.
Patients with advanced HCC are generally immunodeficient, making them susceptible to pneumonia [5]. Carcinomatous lymphangitis should also be considered in patients with malignancy who develop respiratory disorders. In the present three cases, dyspnea was associated with increased serum KL-6 or SP-D concentrations, and the results of blood and sputum examinations suggested that major infection was unlikely. Chest CT showed typical patterns of extensive bilateral interstitial lung disease in all patients, which was distinguishable from carcinomatous lymphangitis [11]. We therefore diagnosed probable sorafenib-induced AIP, although other conditions could not be completely excluded.
Case 1 had a positive DLST, suggesting an allergic reaction to sorafenib. Early discontinuation of sorafenib and administration of PSL resulted in rapid improvement of his respiratory function, supporting allergy as the cause of his AIP. In case 2, we made a definitive diagnosis of AIP by bronchoscopy. The absence of pus in his bronchi, and increased LDH and lymphocytes in his BAL fluid with no evidence of pathogens, helped us to rule out major infections and diagnose AIP. In case 3, it was difficult to conclusively rule out an infectious cause. However, the findings in this case were strongly suggestive of sorafenib-induced AIP.
The characteristics of, and risk factors for, sorafenib-induced AIP are not well known. Our three cases were all men aged over 75 years, and were all smokers (Brinkman index approximately 1,400 in case 1, 400 in case 2, and 800 in case 3). In addition, pretreatment respiratory function testing did not show restrictive lung disease in any of the cases (percent predicted vital capacity 47 % in case 1, 76 % in case 2, and 58 % in case 3). However, pretreatment chest CT did not show evidence of interstitial lung disease, and none of the patients had respiratory symptoms.
Several studies have reported the risk factors for gefitinib-induced AIP in patients with unresectable lung cancer [12, 13]. According to the West Japan Thoracic Oncology Group report on 1,661 patients with lung cancer who were treated with gefitinib, AIP is associated with male sex (odds ratio [OR]: 3.9), smoking (OR: 4.51), and a past history of interstitial pneumonia or respiratory disease (OR: 2.83) [13]. We observed almost the same factors in our three cases.
Table 1 shows the reported cases of sorafenib-induced AIP to date. Although results of pretreatment respiratory function testing were not reported in previous cases, one previous case had a history of interstitial lung disease. The time from initiation of sorafenib therapy to onset of respiratory failure varied from 5 days to 1 year. In all cases, chest X-ray or CT showed GGOs. Our case 2 is the first case in which bronchoscopy was performed. Five of the six cases received steroid therapy.
Drug-induced pneumonia is usually diagnosed by physical examination and X-ray and CT findings. Typical early symptoms of AIP are cough, fever, and dyspnea. Fine crackles can be heard on chest auscultation. However, drug-induced lung disease cannot be definitively diagnosed by physical, laboratory, and imaging findings. Chest X-rays at presentation are likely to miss or underestimate lung disease, but chest CT may be more useful. It is recommended that steroid therapy should be started as soon as possible [14]. In case 1, we diagnosed AIP by CT on the first day of respiratory symptoms and started steroid therapy immediately, whereas in cases 2 and 3 steroid therapy was started a few days after the onset of respiratory symptoms. In the reported fatal case of sorafenib-induced AIP in a patient with RCC, steroid therapy was started 12 days after discontinuing sorafenib. These cases indicate the importance of early diagnosis and immediate initiation of steroid therapy in patients with sorafenib-induced AIP.
The mechanisms of sorafenib-induced interstitial lung disease remain unclear. In general, drug-induced lung disease may be immune-mediated or may result from direct toxicity [15]. The present cases may reflect these two mechanisms. Case 1 had a positive DLST and case 2 had a high serum level of IgE, suggesting an immune-mediated mechanism or acute allergic reaction. Case 3 may have resulted from direct toxicity.
Recently, Myung et al. [7] suggested that sorafenib-induced pulmonary toxicity might be related to its ability to inhibit the vascular endothelial growth factor (VEGF) signaling pathway. Several studies have reported on the relationship between regulation of VEGF and AIP [16–19]. VEGF plays a role in the maintenance of structure and function of alveolar (and other) capillaries. A decrease in the amount or activity of VEGF leads to apoptosis of bronchoalveolar cells, resulting in remodeling of the pulmonary architecture and honeycomb changes in lung structure. Many studies have reported a reduction in the amount of intrapulmonary VEGF in the early stages of lung injury, and normalization of intrapulmonary VEGF after recovery in patients with acute respiratory distress syndrome [16–19].
Sorafenib treatment, which suppresses VEGF, might induce remodeling of bronchoalveolar structures resulting in AIP. Although there are several hypotheses regarding the mechanisms of sorafenib-induced AIP, the pathways remain unclear [7, 8]. Further studies of the molecular mechanisms responsible for sorafenib-induced lung injury are required, and methods of preventing and managing such injury need further investigation.
Conclusion
We report here three cases of sorafenib-induced AIP in patients with advanced HCC. If dyspnea, cough, and high-grade fever develop during sorafenib treatment, AIP should be considered and appropriate investigation and treatment should be initiated as soon as possible. Older age, male sex, smoking history, and a history of lung disease may be risk factors for sorafenib-induced AIP. Further studies on the risk factors and the molecular mechanisms responsible for sorafenib-induced lung injury are needed.
References
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, SHARP Investigators Study Group, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–300.
Miyagawa N, Akaza H. Sorafenib (Nexavar). Gan To Kagaku Ryoho. 2009;36:1029–33.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
Nishikawa H, Osaki Y, Kita R, Kimura T. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma in Japan. Cancer. 2012;4:165–83.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Myung HJ, Jeong SH, Kim JW, Kim HS, Jang JH, Yoon HI, et al. Sorafenib-induced interstitial pneumonitis in a patient with hepatocellular carcinoma: a case report. Gut Liver. 2010;4:543–6.
Ide S, Soda H, Hakariya T, Takemoto S, Ishimoto H, Tomari T, et al. Interstitial pneumonia probably associated with sorafenib treatment: an alert of an adverse event. Lung Cancer. 2010;67:248–50.
Bayer HealthCare Japan. Safety information for Nexabar™ 200 mg tablets. December 2008 (in Japanese).
Horiuchi-Yamamoto Y, Gemma A, Taniguchi H, Inoue Y, Sakai F et al. Drug-induced lung injury associated with sorafenib: analysis of all-patient post-marketing surveillance in Japan. Int J Clin Oncol. 2012;30.
Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27:595–615.
Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303–6.
Gemma A. Drug-induced interstitial lung diseases associated with molecular-targeted anticancer agents. J Nippon Med Sch. 2009;76:4–8.
American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Am J Respir Crit Care Med. 2002;165:277–304.
Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res. 2012;13(1):39.
Papaopannou AI, Konstikas K, Kollia P, Gourgoulianis KI. Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir Res. 2006;7:128.
Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–77.
Mura M, Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol. 2004;97:1605–17.
Richter AG, Maughan EO, Perkins GD, Nathami N, Thickett DR. VEGF levels in pulmonary fibrosis. Thorax. 2005;60:171–3.
Sobin LH, Gospodarowitz MK, Wittekind C. TNM classification of malignant tumours. 7th ed. UICC InternationalUnion Against Cancer;2009.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Takeda, H., Nishikawa, H., Iguchi, E. et al. Sorafenib-induced acute interstitial pneumonia in patients with advanced hepatocellular carcinoma: report of three cases. Clin J Gastroenterol 5, 407–412 (2012). https://doi.org/10.1007/s12328-012-0339-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-012-0339-9