Abstract
Introduction
Given the lack of real-world data on oral semaglutide use outside clinical trials, the purpose of this study was to describe dose, prescriber specialty, and change in hemoglobin A1c (HbA1c) after 6 months of oral semaglutide treatment for patients with type 2 diabetes mellitus (T2DM).
Methods
This was a retrospective study among adult patients with T2DM with ≥ 1 claim for oral semaglutide between November 1, 2019`1–June 30, 2020. Patients had continuous health plan enrollment ≥ 12 months prior to (pre-index) and ≥ 6 months following (post-index) the date of the first oral semaglutide claim (index). Dose at initiation and specialty of the prescribing provider were captured. Change in HbA1c between the last post- and pre-index HbA1c measurement was calculated. Patients were stratified by pre-index HbA1c ≥ 9% (poorly controlled) and HbA1c < 9%.
Results
A total of 744 HbA1c < 9% and 268 poorly controlled patients were included in the study. Most patients had an initial oral semaglutide dose of 7 mg (49.3%) or 3 mg (42.9%), prescribed most frequently by a primary care provider (27.8%). Mean HbA1c reduction was 0.8% (p < 0.001). Patients with poorly controlled T2DM had greater HbA1c reductions than patients with HbA1c < 9% (2.0% versus 0.4%, p < 0.001). Patients persistent with oral semaglutide (≥ 90 days continuous treatment) had a mean HbA1c reduction of 0.9% (p < 0.001); persistent patients with poorly controlled T2DM had a mean reduction of 2.5%.
Conclusions
Patients with T2DM in this study experienced significant reductions in HbA1c within 6 months following initiation of oral semaglutide. Patients with a higher starting HbA1c experienced greater HbA1c reductions. The initial dose of oral semaglutide was higher than prescribing instructions indicated for more than half of the study patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Oral semaglutide, the first oral GLP-1 receptor agonist, was approved by the Food and Drug Administration in 2019 for glycemic control among patients with type 2 diabetes mellitus, particularly those who have comorbid cardiovascular or kidney disease |
Clinical trials have shown significant decreases in hemoglobin A1c among patients taking oral semaglutide; however, there is a lack of data on the real-world use and HbA1c benefits outside of clinical trial populations |
The objectives of this study were to describe the dose, prescriber specialty, and change in hemoglobin A1c after 6 months of oral semaglutide therapy among patients with type 2 diabetes mellitus |
What was learned from the study? |
Despite evidence of advanced type 2 diabetes mellitus and high rates of hypertension, lipid disorders, and other nutritional, endocrine, and metabolic disorders, patients in this study had a mean hemoglobin A1c reduction of 0.8%; this reduction was higher among patients with poorly controlled type 2 diabetes mellitus (hemoglobin A1c ≥ 9.0%) and those persistent with oral semaglutide treatment |
Future research is needed to understand the relationship among provider specialty, hemoglobin A1c values, and prescribing patterns among patients with type 2 diabetes mellitus |
Introduction
An estimated 130 million people are living with diabetes or prediabetes in the US, and 90%–95% of them have type 2 diabetes mellitus (T2DM) [1]. Glycated hemoglobin (HbA1c) is a measure of glycemic control and a strong predictor for complications in patients with T2DM [2,3,4]. For each 1% reduction in HbA1c among patients with T2DM, risk reductions of 43% in amputation or death from peripheral vascular disease, 37% in microvascular complications, 21% in any diabetes-related end point and diabetes-related mortality, 19% in cataract extractions, 16% in heart failure, 14% in myocardial infarctions and all-cause mortality, and 12% in stroke have been reported [4].
Achieving and maintaining proper glycemic control are challenging, particularly when factoring in disease progression and comorbidity burden. In most patients, an HbA1c goal of < 7% is sought given the benefits of reducing or preventing diabetes-related complications; however, an HbA1c goal of < 8% in some patients may be necessary to balance these benefits with the risk of hypoglycemia [5, 6]. Given the chronic nature of T2DM, the HbA1c goal may change over the disease course based on patient factors including comorbidities, vascular complications, life expectancy, patient preference, and available resources and support [7, 8]. Between 2006 and 2013, the treatment landscape shifted to newer glucose-lowering agents; however, the proportion of patients with an HbA1c < 7% decreased from 56.4 to 54.2%, while the proportion of patients with an HbA1c ≥ 9% increased from 9.9 to 12.2% [9].
To maintain optimal glycemic control in patients with T2DM, lifestyle modifications and pharmacotherapy with oral non-insulin anti-diabetic agents (NIADs) or insulin are needed [10]. The agent selected as first-line therapy depends on comorbidities and patient management needs, but typically includes metformin, in addition to lifestyle modifications. Other agents, including glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT-2is)—with or without metformin—may be used as initial therapy for those with T2DM with or at high risk for atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease. As T2DM is a progressive disease, treatment intensification with combination therapy may be necessary to maintain glycemic targets. In a meta-analysis, NIADs decreased HbA1c levels by 0.5–1.25% [11]. A 1% higher baseline HbA1c level was predictive of a 0.5% greater reduction in HbA1c levels after 6 months of NIAD therapy.
Oral semaglutide was approved by the Food and Drug Administration in 2019 as the first oral GLP-1 RA, providing a new treatment option to patients with comorbid cardiovascular or kidney disease who are unable or unwilling to self-administrator an injectable agent. In clinical trials, patients with an oral semaglutide dose escalated to 14 mg for 26 weeks had a mean HbA1c decrease of 1.0–1.4% [12,13,14,15,16,17]. Given the lack of data on the real-world use of oral semaglutide and the HbA1c benefits seen outside of a clinical trial population, the objectives of this study were to describe the dose, prescriber specialty, and change in HbA1c after 6 months of oral semaglutide treatment for patients with T2DM.
Methods
Data Source
This was a retrospective study that used medical claims, pharmacy claims, and enrollment information from November 1, 2018, to December 31, 2020 (study period) from commercial and Medicare Advantage health plan members in the Optum Research Database (ORD). The ORD is a custom research database owned by Optum’s parent company, and use of the data for this study was overseen by a data governing board comprised of data providers, data users, and compliance experts. Relevant medical claims were identified using International Classification of Diseases, 10th Revision, Clinical Modification diagnosis and procedure codes, Current Procedural Terminology Codes, and Healthcare Common Procedure Coding System codes and revenue codes. Relevant outpatient pharmacy codes were identified using National Drug Codes. Outpatient laboratory test results were defined using standard LOINC coding. This study used protected health information that had been de-identified in accordance with Health and Human Services Privacy Rule’s requirements for de-identification codified at 45 C.F.R § 164.514(b) and was not subject to an IRB review.
Study Sample Selection
Patients with ≥ 1 claim for oral semaglutide between November 1, 2019, and June 30, 2020, were included in the study. The date of the first oral semaglutide claim was the index date. Patient were required to have continuous health plan enrollment with medical and pharmacy benefits for ≥ 12 months prior to and including the index date (pre-index period) and ≥ 6 months following the index date (post-index period), ≥ 1 diagnosis code for T2DM during the pre- or post-index periods, and an age of ≥ 18 years as of the index year. Patients were excluded from the study if they had evidence of pregnancy during the pre- or post-index periods or if they did not have a recorded HbA1c value during the pre-index period.
Pre-index Demographic and Clinical Characteristics
Patient demographic and clinical characteristics measured during the pre-index period included age, gender, insurance type, geographic region, Charlson comorbidity index [18, 19], and frequently diagnosed comorbid conditions operationalized by the Agency for Healthcare Research and Quality [20].
Pre-index Medication Classes
The proportion of patients with ≥ 1 pharmacy claim and the number of agents filled based on pre-specified American Hospital Formulary Service (AHFS) classes [21] during the pre-index period were documented.
Outcomes
The designated dose on the index oral semaglutide pharmacy claim and the prescriber specialty (identified via the specialty code on the pharmacy claim) were identified. Maintenance dose was defined as the designated dose with the largest proportion of days covered, starting on the index date. The change in HbA1c was calculated as the last HbA1c measured during the post-index period minus the last HbA1c value measured during the pre-index period among patients with an HbA1c value in both the pre- and post-index periods and separately among patients who were persistent with therapy (i.e., patients with continuous oral semaglutide treatment for ≥ 90 days with an HbA1c value ≥ 90 days after the index date).
Analysis
Patients were stratified by their last measured pre-index HbA1c value and were considered poorly controlled if they had an HbA1c ≥ 9.0%. The HbA1c stratifications were based on the Healthcare Effectiveness Data and Information Set cutoff of > 9% for poor control [22]. The focus of the analysis was on the poorly controlled cohort, and the HbA1c < 9% cohort was included for completeness. Numbers and percentages were provided for dichotomous and polychotomous variables; means and standard deviations were provided for continuous variables. Two sample t-tests and Pearson chi-square tests were used for comparisons among patients with poorly controlled T2DM and patients with a pre-index HbA1c < 9% and whether the pre-index to post-index change in HbA1c differed from zero.
Results
Pre-index Demographic and Clinical Characteristics
A total of 1012 patients were included in the study population, of which 744 had an HbA1c < 9% and 268 had poorly controlled T2DM during the pre-index period (Fig. 1). Patients had a mean (standard deviation [SD]) age of 59.0 (11.8) years, 50.0% were male, and the majority were commercially insured (63.5%) and lived in the South (68.7%) (Table 1). The mean Charlson comorbidity score was 1.2 (SD = 1.5), and frequently diagnosed comorbidities included lipid metabolism disorders (86.0%), hypertension (81.5%), diabetes with (75.8%) and without (84.5%) complications (patients could have both diagnosis codes), and other nutritional, endocrine, or metabolic disorders (70.2%). Patients with poorly controlled T2DM were slightly younger (57.6 versus 59.5 years, p = 0.030), less frequently lived in the Midwest (7.5% versus 13.7%, p = 0.007), and were more likely to have diabetes with complications (85.8% versus 72.2%, p < 0.001) than patients with HbA1c < 9%.
Pre-index Medication Classes
The most frequently filled AHFS medication classes during the pre-index period are shown in Fig. 2. More than half of patients had ≥ 1 fill for antihypertensive agents (79.6%), lipid-lowering agents (76.9%), renin-angiotensin-aldosterone (RAAS) inhibitors (69.3%), and antibacterial agents (52.1%). Patients filled a mean (SD) of 12.0 (6.1) different medication classes during the pre-index period, with a mean (SD) of 13.4 (6.9) unique compounds filled.
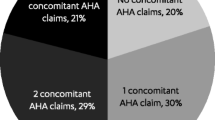
Patients frequently had ≥ 1 fill for metformin (76.9%), approximately one-third had ≥ 1 fill for a SGLT-2 (34.9%) or a sulfonylurea (30.1%), and one-fourth had ≥ 1 fill for a dipeptidyl peptidase-4 inhibitor (DPP-4i, 25.5%), GLP-1 RA (25.1%) or insulin (24.4%) (Fig. 3). Only 5.1% of patients had no pre-index anti-diabetic treatment. Patients with poorly controlled T2DM were more likely to have fills for metformin (82.1% versus 75.0%, p = 0.018), sulfonylureas (36.2% versus 28.0%, p = 0.012), and insulin (37.3% versus 19.8%, p < 0.001) and were less likely to have no pre-index anti-diabetic treatment (1.9% versus 6.3%, p = 0.005).
Diabetic medication classes filled during the pre-index period (including on the index date). *p < 0.05. 1Excludes oral semaglutide. DPP-4i dipeptidyl peptidase-4 inhibitor, GLP-1 RA glucagon-like peptide-1 receptor agonist, HbA1c hemoglobin A1c, SLGT-2i sodium-glucose cotransporter 2 inhibitor, T2DM type 2 diabetes mellitus, TZD thiazolidinedione
Index Oral Semaglutide Dose and Prescriber
On the index date, most patients were prescribed a semaglutide dose of either 3 mg (42.9%) or 7 mg (49.3%), with few patients having an index semaglutide fill for 14 mg (7.8%). Evidence of multiple index doses was found in 3.5% of patients (Table 2). More than one-fourth of patients were prescribed their index semaglutide dose by a primary care provider (27.8%), followed closely by an internal medicine provider (22.8%), endocrinologist (22.1%), and “other” provider type (20.5%). Patients with poorly controlled T2DM were significantly less likely to have an endocrinologist as their prescriber (16.0% versus 24.3%, p = 0.005) and more likely to have “other” provider type as their prescriber (24.6% versus 19.0%, p = 0.048) than patients with an HbA1c < 9%. More than half of patients (53.8%) had a semaglutide maintenance dose of 7 mg.
Change in HbA1c from the Pre-index to Post-index Period
A total of 667 patients (65.9%) had an HbA1c measurement in both the pre- and post-index periods, with an average of 185.7 days between measurements (Table 3). The mean (SD) HbA1c change from the last pre-index measurement to the last post-index measurement was a reduction of 0.8% (1.5%) (p < 0.001). Over one-third (35.5%) of patients had an HbA1c reduction ≥ 1%. Patients with poorly controlled T2DM had a significantly greater reduction in HbA1c than patients with a pre-index HbA1c < 9% (2.0% versus 0.4%, p < 0.001). A total of 67.4% of patients with poorly controlled T2DM had a mean HbA1c reduction ≥ 1% compared with 24.2% of patients with a pre-index HbA1c < 9% (p < 0.001).
Among patients who were persistent with oral semaglutide therapy (n = 295), the mean (SD) HbA1c reduction was 0.9% (1.4%) (p < 0.001) and 39.0% had an HbA1c reduction ≥ 1% (Table 3). Persistent patients with poorly controlled T2DM had a significantly greater reduction in HbA1c than patients with a pre-index HbA1c < 9% (2.5% versus 0.5%, p < 0.001). A total of 80.6% of persistent patients with poorly controlled T2DM had a mean reduction in HbA1c ≥ 1% compared with 26.8% of patients with a pre-index HbA1c < 9% (p < 0.001).
Discussion
The goal of this study was to describe medication dose, prescriber specialty, and change in HbA1c among patients with T2DM in a real-world clinical setting within 6 months following initiation of oral semaglutide. Patients in this study had an overall reduction in HbA1c of 0.8%. Patients with poorly controlled T2DM experienced significantly greater HbA1c reductions than patients with a pre-index HbA1c < 9%. Patients who were persistent with oral semaglutide had a mean HbA1c reduction of 0.9%. Persistent patients with poorly controlled T2DM had a significantly greater HbA1c reduction than persistent patients with a pre-index HbA1c < 9%.
Patients in this study were medically complex and in the later stages of T2DM. Approximately 80% of patients with diabetes suffer from additional chronic diseases requiring frequent use of polypharmacy [23, 24], which can lead to suboptimal glycemic control and an increased risk of long-term diabetes-related complications [25]. In this study, > 70% of patients had lipid disorders, hypertension, or other nutritional, endocrine, or metabolic disorders in addition to T2DM. Patients filled a mean of 12 different medication classes and more than 13 different compounds during the 12-month pre-index period. Interactions between multiple drugs and diseases can complicate optimal management and control of chronic conditions, including drug-drug interactions and adverse drug reactions [26, 27]. The high level of health complications among study patients may exacerbate the risk of known negative health outcomes, including poor glycemic control [28], hypoglycemic events [29, 30], syncope [30], poor quality of life [31, 32], need for inpatient care [30], and death [30, 33], highlighting that more effective T2DM treatments, not just more treatments, are needed.
Despite evidence of advanced T2DM and medical complexity, patients in this study experienced a significant reduction in HbA1c within 6 months following initiation with oral semaglutide. Patients with poorly controlled T2DM had an HbA1c reduction of 2.0%, which increased to 2.5% among patients persistent with oral semaglutide. In a trial of 14 mg oral semaglutide among patients on metformin with an HbA1c of 7.0–10.5%, patients experienced an HbA1c reduction of 1.3% after 26 weeks of semaglutide treatment [13]. In a similar trial among patients with an HbA1c of 7.0–9.5%, patients experienced a 1.2% reduction in HbA1c after 26 weeks of oral semaglutide treatment (target dose of 14 mg) [15]. The greater HbA1c reductions among patients with poorly controlled T2DM in this study were consistent with a previously published study by Sherifali et al., who reported a 1% higher baseline HbA1c predicted a 0.5% greater HbA1c reduction after 6 months of NIAD treatment [11].
Prescribing instructions recommend a starting dose of 3 mg of oral semaglutide once daily, with a dose increase to 7 mg after 30 days. If further escalation is needed, patients can increase to 14 mg once daily after 30 days on the 7 mg dose. The 3 mg dose is not intended as a therapeutic dose but rather a starting dose to mitigate gastrointestinal adverse events [34]. In this study, 57.1% of patients were initiated at a dose higher than 3 mg, with most patients initiated on a 7 mg dose. This may be partially explained by the fact that some of the 25.1% of patients prescribed a GLP-1 during the pre-index period may have been prescribed subcutaneous semaglutide and would have started an oral dose of 7 mg or 14 mg as recommended [34, 35]. In clinical trials of oral semaglutide, patients were dose escalated to 14 mg [13, 15]; however, only 16.4% of patients in this study had a maintenance dose of 14 mg and 29.8% remained on a non-therapeutic maintenance dose of 3 mg. This may explain the lower HbA1c reduction among the overall patient population compared to those seen in clinical trials. Real-world studies are needed to evaluate HbA1c reduction among patients on oral semaglutide who reach a therapeutic dose. Half of patients were prescribed oral semaglutide by a primary care or internal medicine provider who may be less aware of the dose titration schedule set forth by the manufacturer than an endocrinologist or diabetes specialist. Previous studies have shown that patients with T2DM have better utilization of diabetes-related process of care measures when treated by endocrinologists or diabetes specialists than by general medicine or family practice providers [36, 37]. Partly at fault is the current structure of the US healthcare system, which does not allow adequate time for primary care providers to address diabetes management in addition to other patient complaints and illnesses given the short time allotted for patient appointments. Given the shortage of endocrinologists and the demand for diabetes-related care, there is a strong need for additional training, with an emphasis on comprehensive diabetes management, for primary care and internal medicine providers [38].
Limitations
Healthcare claims data are collected for service payment and not research; thus, several limitations are inherent in this type of study. Medication use was measured using pharmacy claims, and patients may not have taken the medication as prescribed. Additionally, medication samples provided to the patient were not included in this study. It is possible that patients with a documented 7 mg index dose may have received 3 mg samples that were not recorded as their actual index dose. As HbA1c laboratory data were not available for all study patients, subpopulations with HbA1c measures were examined to mitigate this limitation. Claims data also do not include clinical data, such as body mass index/weight, or social determinants of health information. Lastly, this study was conducted in a large US-managed care population and may not be representative of all patients with T2DM.
Conclusions
Despite the medical complexity and the later disease stage, patients with T2DM in this study experienced significant reductions in HbA1c within 6 months following initiation of oral semaglutide. Patients with a higher HbA1c during the pre-index period experienced greater HbA1c reductions. The initial dose of oral semaglutide was higher than prescribing instructions indicated for more than half of the study patients. Future research is needed to understand the relationship among provider specialty, HbA1c values, and prescribing patterns in patients with T2DM.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the proprietary elements contained in the database, which are owned by Optum. The disclosure of this data to third part clients assumes certain data security and privacy protocols are in place and that the third-party client has executed Optum’s standard license agreement which includes restrictive covenants governing the use of the data.
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report website. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Updated June 29, 2022. Accessed Sep 29, 2022.
Laiteerapong N, Ham SA, Gao Y, et al. The Legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the diabetes and aging study). Diabetes Care. 2019;42(3):416–26.
Little RR, Rohlfing C, Sacks DB. The national glycohemoglobin standardization program: over 20 years of improving hemoglobin A1c measurement. Clin Chem. 2019;65(7):839–48.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–12.
American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S73–84.
Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168(8):569–76.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40(4):468–75.
American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 9 Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125–43.
Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010;33(8):1859–64.
Aroda VR, Rosenstock J, Terauchi Y, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42(9):1724–32.
Rodbard HW, Rosenstock J, Canani LH, et al. Oral Semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. 2019;42(12):2272–81.
Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–80.
Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50.
Mosenzon O, Blicher TM, Rosenlund S, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–27.
Zinman B, Aroda VR, Buse JB, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: the PIONEER 8 trial. Diabetes Care. 2019;42(12):2262–71.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
Bayliss EA, Ellis JL, Shoup JA, Zeng C, McQuillan DB, Steiner JF. Association of patient-centered outcomes with patient-reported and ICD-9-based morbidity measures. Ann Fam Med. 2012;10(2):126–33.
Agency for Healthcare Research and Quality. Clinical classification software (CCS) for ICD-10-CM. https://www.hcup-us.ahrq.gov/toolssoftware/ccs10/ccs10.jsp. Updated Nov 5, 2019. Accessed Feb 22, 2023.
Cleveland CB. Advantages of a universal coding and classification system for drugs. Classification and coding system of the American Hospital Formulary Service. Am J Hosp Pharm. 1966;23(2):95–7.
National Committee for Quality Assurance. Comprehensive Diabetes Care. https://www.ncqa.org/hedis/measures/comprehensive-diabetes-care/. Accessed Feb 2, 2023.
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43.
Marengoni A, Rizzuto D, Wang HX, Winblad B, Fratiglioni L. Patterns of chronic multimorbidity in the elderly population. J Am Geriatr Soc. 2009;57(2):225–30.
van Oort S, Rutters F, Warle-van Herwaarden MF, et al. Characteristics associated with polypharmacy in people with type 2 diabetes: the Dutch Diabetes Pearl cohort. Diabet Med. 2021;38(4): e14406.
Oktora MP, Alfian SD, Bos HJ, et al. Trends in polypharmacy and potentially inappropriate medication (PIM) in older and middle-aged people treated for diabetes. Br J Clin Pharmacol. 2021;87(7):2807–17.
Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf. 2007;30(10):911–8.
McCracken R, McCormack J, McGregor MJ, Wong ST, Garrison S. Associations between polypharmacy and treatment intensity for hypertension and diabetes: a cross-sectional study of nursing home patients in British Columbia, Canada. BMJ Open. 2017;7(8): e017430.
Noale M, Veronese N, Cavallo Perin P, et al. Polypharmacy in elderly patients with type 2 diabetes receiving oral antidiabetic treatment. Acta Diabetol. 2016;53(2):323–30.
Kabue S, Liu V, Dyer W, Raebel M, Nichols G, Schmittdiel J. Identifying common predictors of multiple adverse outcomes among elderly adults with type-2 diabetes. Med Care. 2019;57(9):702–9.
Yang YC, Lin MH, Wang CS, et al. Geriatric syndromes and quality of life in older adults with diabetes. Geriatr Gerontol Int. 2019;19(6):518–24.
Al-Musawe L, Torre C, Guerreiro JP, et al. Polypharmacy, potentially serious clinically relevant drug-drug interactions, and inappropriate medicines in elderly people with type 2 diabetes and their impact on quality of life. Pharmacol Res Perspect. 2020;8(4): e00621.
Forbes A, Murrells T, Sinclair AJ. Examining factors associated with excess mortality in older people (age >/= 70 years) with diabetes—a 10-year cohort study of older people with and without diabetes. Diabet Med. 2017;34(3):387–95.
Rybelsus. Prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209637s003lbl.pdf. Updated Jan 2020. Accessed Oct 03, 2022.
Almandoz JP, Lingvay I, Morales J, Campos C. Switching between glucagon-like peptide-1 receptor agonists: rationale and practical guidance. Clin Diabetes. 2020;38(4):390–402.
Ho M, Marger M, Beart J, Yip I, Shekelle P. Is the quality of diabetes care better in a diabetes clinic or in a general medicine clinic? Diabetes Care. 1997;20(4):472–5.
Chin MH, Zhang JX, Merrell K. Specialty differences in the care of older patients with diabetes. Med Care. 2000;38(2):131–40.
Healy AM, Shubrook JH, Schwartz FL, Cummings DM, Drake AJ 3rd, Tanenberg RJ. Endocrinologists’ opinions of diabetology as a primary care subspecialty. Clin Diabetes. 2018;36(2):168–73.
Medical Writing and Editorial Assistance
Authors would like to acknowledge Kim McNiff, Optum, for analysis work, Sarah Hague, Optum, for project management, and Deja Scott-Shemon, Optum, for medical writing services. Funding for medical writing services was provided by Novo Nordisk, Inc.
Funding
This study and the journal’s Rapid Service Fee was funded by Novo Nordisk, Inc.
Author information
Authors and Affiliations
Contributions
Conceptualization and study design was conducted by Monica Frazer, Caroline Swift, Sarah Alvarez, Josh Noone, and Mico Guevarra. The study methodology was determined by Monica Frazer, Caroline Swift, Noelle N. Gronroos, Tyler J. Dunn, and Josh Noone. Acquisition and analysis of the data were conducted by Andrew Sargent, Michael Leszko, and Erin Buysman. Caroline Swift, Noelle N. Gronroos, and Tyler J. Dunn interpreted the findings. All authors read and approved the final manuscript and reviewed and commented on all previous versions.
Corresponding author
Ethics declarations
Conflict of Interest
Caroline Swift, Sarah Alvarez, Tyler J. Dunn, Josh Noone, and Mico Guevarra are employees of Novo Nordisk. Monica Frazer was an employee of Optum at the time the study was conducted and is currently employed at QualityMetric. Andrew Sargent, Michael Leszko, Noelle N. Gronroos, and Erin Buysman are employees of Optum.
Ethical Approval
This study used protected health information that had been de-identified in accordance with Health and Human Services Privacy Rule’s requirements for de-identification codified at 45 C.F.R § 164.514(b) and was not subject to an IRB review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Frazer, M., Swift, C., Gronroos, N.N. et al. Real-World Hemoglobin A1c Changes, Prescribing Provider Types, and Medication Dose Among Patients with Type 2 Diabetes Mellitus Initiating Treatment with Oral Semaglutide. Adv Ther 40, 5102–5114 (2023). https://doi.org/10.1007/s12325-023-02677-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02677-w