Abstract
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin disease characterized by heterogeneous clinical phenotypes and high symptom burden, especially through itch. Baricitinib (BARI), an oral Janus Kinase 1/2 inhibitor, is approved in Europe, Japan, and other countries, for treatment of adults with moderate-to-severe AD who are candidates for systemic therapy. This post hoc analysis of a Phase 3 topical corticosteroid (TCS) combination therapy trial (BREEZE-AD7) aims to characterize patients who might benefit most from BARI.
Method
Classification and regression tree (CART) analysis was used to identify baseline predictors for patients treated with BARI 4-mg, who achieved ≥ 75% improvement in Eczema Area and Severity Index (EASI75), or EASI75 or Itch Numerical Rating Scale (NRS) ≥ 4-point improvement at week 16 (responders), versus non-responders. Subgroup efficacy analyses were performed based on identified predictor variables, combined with Itch NRS < 7/ ≥ 7. Missing data were imputed as non-responder.
Results
Baseline body surface area (BSA) was identified by CART as strongest variable predicting response to BARI at week 16, with a cut-off around 40% (BSA ≤ 40%). When combining BSA with itch severity, highest response rates were achieved by BARI patients with BSA ≤ 40%/Itch NRS ≥ 7 at baseline. In this subgroup, 69% and 58% of patients treated with BARI 4-mg achieved EASI75 and Itch NRS ≥ 4-point response at week 16, respectively. While these response rates were 65% and 50% for BARI 4-mg patients with baseline BSA ≤ 40%/Itch NRS < 7, they were 33% and 11% in BSA > 40%/Itch NRS < 7, and 32% and 49% in BSA > 40%/Itch NRS ≥ 7 subgroups, respectively.
Conclusion
Using a machine learning approach, patients with moderate-to-severe AD and a BSA affecting 10–40% and Itch NRS ≥ 7 were characterized as likely to benefit most from BARI 4-mg TCS combination therapy. This was confirmed by subgroup analyses, which showed that these patients are most likely to show favorable response rates in improving AD signs and symptoms, specifically itch, after 16 weeks of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Atopic dermatitis is a chronic and relapsing inflammatory skin disease characterized by a complex pathophysiology and clinical phenotype, usually treated under the ‘‘one-size-fits-all’’ approach. |
Baricitinib, an oral Janus Kinase 1/2 inhibitor, is approved in Europe, Japan and other countries, for treatment of adults with moderate-to-severe atopic dermatitis who are candidates for systemic therapy. |
What was learned from the study? |
Using a machine learning approach, this post hoc analysis identified and characterized patients who are likely to benefit most from baricitinib. |
Patients with moderate-to-severe atopic dermatitis and body surface area affecting 10–40% and severe itch were characterized as likely to benefit most from baricitinib 4-mg topical corticosteroid combination therapy, and were most likely to show favorable response rates in improving atopic dermatitis signs and symptoms, specifically itch. |
Identifying patients likely to benefit most from baricitinib 4-mg treatment may allow precision medicine when making individual treatment decisions. |
Observational studies and atopic dermatitis registries will provide critical data sources to confirm whether this clinical tailoring approach holds true in a real-world setting. |
Introduction
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease characterized by a complex pathophysiology and clinical phenotype. Despite the heterogeneity in the disease course and clinical presentation of signs and symptoms associated with AD, it is considered a single disease entity, usually treated under the ‘‘one-size-fits-all’’ approach [1]. This approach, however, does not always seem to be appropriate due to the diverse disease phenotypes and varied therapeutic response experienced by patients [2].
The majority of patients with AD experience chronic itch, making it a defining feature of AD, which substantially impacts physical, social, and psychological well-being [3]. Itch and physician-assessed severity can be used to describe unique AD phenotypes, one of which being the “itch-dominant” phenotype that is characterized by mild-to-moderate lesions and severe itch [4]. Potential differences in effects of various treatments on these AD phenotypes are not fully understood; therefore, further research is needed to enable optimal treatment selection for patients with different levels of itch and degree of skin involvement.
Baricitinib (BARI) was the first oral selective Janus kinase 1/2 inhibitor approved in Europe, Japan, and other countries for the treatment of moderate-to-severe AD in adult patients who are candidates for systemic therapy. While BARI 2-mg is reserved for special patient populations, the recommended starting dose is 4 mg [5], which has a rapid effect in improving AD signs and symptoms, specifically on itch. Along with BARI, several other systemic treatments are recommended for patients not sufficiently controlled on topical therapies by the most recent European Guideline for atopic eczema (dermatitis) [6].
While treatment decisions should be made on an individual patient level, specific patient characteristics determining the optimal response to those treatments, including BARI, remain elusive. Therefore, using a machine learning approach on data from the Phase 3 topical corticosteroid (TCS) combination therapy trial BREEZE-AD7, the objective of this post hoc analysis was to identify and characterize patients who are likely to benefit most from BARI 4-mg, and to propose a clinical tailoring approach, taking into account unique AD phenotypes.
Methods
Study Design
This post hoc analysis included data from patients enrolled in the TCS combination therapy trial BREEZE-AD7 (NCT03733301) (n = 329). A detailed study design has been previously published [7]. In summary, BREEZE-AD7 was a 16-week randomized, double-blind, parallel-group, placebo-controlled trial. Patients were randomized 1:1:1 to receive once-daily placebo, BARI 2-mg, or BARI 4-mg, stratified by baseline disease severity and geography. Moderate- and/or low-potency TCS use was allowed on active lesions, with topical calcineurin inhibitors and/or crisaborole permitted as alternative on sensitive areas.
Participants were ≥ 18 years old, were diagnosed with AD ≥ 12 months prior to screening, and had a history of inadequate response to topical treatments. Moderate-to-severe AD was defined by an Eczema Area and Severity Index (EASI) score ≥ 16, a Validated Investigator’s Global Assessment of AD (vIGA-AD) score ≥ 3, and ≥ 10% body surface area (BSA) at screening and baseline.
Prior to enrollment, patients provided written informed consent. The study was conducted with the approval of each center’s institutional review board or independent ethics committee and in accordance with the guiding principles of the Declaration of Helsinki.
Identification of Responders to BARI and Definition of Subgroups
A two-step analysis approach was considered. First, a classification and regression tree (CART) [8] analysis was used to identify subgroups of patients most likely to benefit from BARI 4-mg. Response to BARI 4-mg was defined as achieving ≥ 75% improvement in EASI (EASI75) or as achieving EASI75 or itch Numerical Rating Scale (NRS) ≥ 4-point improvement from baseline at week 16. Second, CART-identified baseline predictors of response were subsequently combined with baseline mild to moderate (Itch NRS < 7) or severe (Itch NRS ≥ 7) itch levels in order to define subgroups that reflect clinically relevant AD phenotypes. Efficacy outcomes will be compared within those clinically relevant AD subgroups.
Efficacy Outcomes
Endpoints analyzed in this post hoc analysis included the proportion of responders who achieved EASI75 and the proportion of patients who achieved a ≥ 4-point improvement from baseline in Itch NRS (among those with Itch NRS ≥ 4 at baseline) over 16 weeks. Additional endpoints included the proportion of patients who achieved Atopic Dermatitis Sleep Scale (ADSS) Item 2 ≥ 1.5 point improvement from baseline (among those with ADSS Item 2 ≥ 1.5 point at baseline).
Statistical Analysis
Baseline patient demographics (age, sex, race, region, and body mass index), disease characteristics (time since AD diagnosis, presence of atopic comorbidities, prior systemic therapy use, prior cyclosporine use, tobacco use, and alcohol use), disease severity measures [EASI total score, EASI head/neck score, BSA derived from EASI, Scoring of Atopic Dermatitis (SCORAD)], patient-reported outcomes [Itch NRS, Patient Orientated Eczema Measure (POEM), Dermatology Life Quality Index (DLQI)], and laboratory parameters [immunoglobulin E (IgE) levels and eosinophil counts] were individually used in the CART model to predict responders (listed in previous paragraph) versus non-responders to BARI 4-mg in TCS combination therapy. CART [R, recursive partitioning, and regression trees (rpart)] [9] was used to identify predictors with ideal cut-offs, as described before [8]. Prediction accuracy including sensitivity, specificity, negative predictive value, and positive predictive value evaluating robustness of identified predictors from CART are reported in the supplement (Supplementary Table 1). As a sensitivity analysis, the same method was applied to identify baseline predictors to BARI 4-mg responders in monotherapy from BREEZE-AD1 and BREEZE-AD2 [10].
Patient demographics and disease characteristics were analyzed descriptively across defined subgroups and were reported as mean ± standard deviation (SD) or percentage (%). Response rates over 16 weeks within those subgroups were analyzed with logistics regression models (cut-off for interaction term was 0.1) by visit. Baseline disease severity (vIGA-AD), continuous version of endpoint at baseline, treatment group, region, baseline subgroup, and treatment-by-baseline subgroup interaction were included as model terms. Missing data were imputed as non-responders, with data after permanent study discontinuation or after rescue (systemic therapies or high-to-ultrahigh potency TCS) considered missing. Statistical significance was not adjusted for multiplicity.
Results
Identification of Baseline Predictors for Clinical Response to BARI 4-mg
Using a machine learning framework (CART), baseline BSA was consistently identified as the strongest variable predicting achievement of EASI75, and EASI75 or itch 4-point improvement response at week 16 in patients receiving BARI 4-mg in TCS combination therapy, with a cut-off at 46% BSA (Fig. 1A, B). Sensitivity analyses using data from patients treated with BARI 4-mg in monotherapy (BREEZE-AD1/2) confirmed BSA as the strongest baseline predictor for BARI 4-mg response at week 16 and established a cut-off at 36% BSA (data not shown). Based on these consistent CART outcomes across studies and response measures, BSA with a threshold of 40% (see model accuracy in Supplementary Table 1) was selected to define subgroups of patients likely to benefit most from BARI 4-mg treatment, combined with different levels of itch [11] to reflect distinct AD phenotypes. The following subgroups were thus defined: (1) BSA 10–40%/Itch NRS < 7, (2) BSA 10–40%/Itch NRS ≥ 7 (‘itch-dominant phenotype’), (3) BSA > 40%/Itch NRS < 7, and (4) BSA > 40%/Itch NRS ≥ 7.
CART-identified tree to predict A EASI75 and B EASI75 or Itch NRS 4-point response from baseline (Itch NRS 4-point) at week 16 for patients treated with baricitinib 4-mg in the topical corticosteroid combination therapy trial BREEZE-AD7. BSA body surface area, CART classification and regression tree, EASI75 ≥ 75% improvement in Eczema Area and Severity Index, NRS Numerical Rating Scale, ITT intent-to-treat
Patient Demographics and Disease Characteristics in Identified Subgroups
Of patients randomized to BARI 4-mg at baseline, patients with BSA 10–40%/Itch NRS < 7 accounted for 18% (n = 20), patients with BSA 10–40%/Itch NRS ≥ 7 for 23% (n = 26), patients with BSA > 40%/Itch NRS < 7 for 19% (n = 21), and patients with BSA > 40%/Itch NRS ≥ 7 for 37% (n = 41) (Table 1). Across subgroups, age and AD disease duration were similar. Mean BSA affected by AD was 28% and 30%, for the BSA 10–40%/Itch NRS < 7 and BSA 10–40%/Itch NRS ≥ 7 subgroups, respectively, while it was 66% and 69%, for the BSA > 40%/Itch NRS < 7 and BSA > 40%/Itch ≥ 7, subgroups, respectively. Mean EASI was 19 and 21, in the BSA 10–40%/Itch NRS < 7 and BSA 10–40%/Itch NRS ≥ 7 subgroups, respectively, and 35 and 41, in the subgroups with BSA > 40% NRS < 7 and BSA > 40%/Itch NRS ≥ 7, respectively. Of note, in the BSA 10–40% subgroups, 15% of those with Itch NRS < 7, and 35% with Itch NRS ≥ 7 had an EASI > 21, reflecting severe AD, and EASI values reaching up to 30.5. All subgroups were classified as severe based on mean SCORAD (SCORAD > 50). Mean Itch NRS for the subgroups was 5 for both BSA 10–40%/Itch NRS < 7 and BSA > 40%/Itch NRS < 7 subgroups, and 8 and 9 for BSA 10–40%/Itch NRS ≥ 7 and BSA > 40%/Itch NRS ≥ 7 subgroups, respectively. Among subgroups, those with Itch NRS ≥ 7 tended to have higher values for sleep disturbance (ADSS Item 2), quality of life impairment (DLQI), and Hospital Anxiety and Depression Scale (HADS) anxiety and depression subscales than respective BSA subgroups with Itch NRS < 7. Patients with BSA 10–40%/Itch NRS ≥ 7 consistently tended to score higher on these PROs than those with BSA > 40%/Itch NRS < 7. Distribution of subgroups, baseline demographics, and disease characteristics were comparable in the placebo arm (Table 1).
Clinical Outcomes for BARI 4-mg Over 16 Weeks in Identified Subgroups
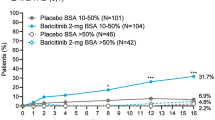
For patients in subgroups with BSA 10–40%, response rates for EASI75 were significantly higher with BARI 4-mg compared to placebo at week 16 (Fig. 2). Significant differences for BARI 4-mg versus placebo were achieved as early as week 2 for patients across BSA 10–40% subgroups. In the BSA 10–40%/Itch NRS ≥ 7 subgroup, EASI75 response at week 16 for BARI 4-mg was 69% and 65% for patients with BSA 10–40%/Itch NRS < 7 relative to 33% and 32% in the BSA > 40%/Itch NRS < 7 and BSA > 40%/Itch NRS ≥ 7 subgroups, respectively, highlighting that patients with BSA 10–40% have the highest probability of achieving EASI75 response and thereby confirming CART predictions.
EASI75 response rate over 16 weeks across identified BSA/Itch NRS subgroups. Proportion of patients achieving a 75% improvement in EASI score with A BSA 10–40%/Itch NRS < 7, B BSA 10–40%/Itch NRS ≥ 7 (itch-dominant phenotype), C BSA > 40%/Itch NRS < 7, and D BSA > 40%/Itch NRS ≥ 7. p-values are based on Fisher’s exact test. *p < 0.05, **p < 0.01, and ***p < 0.001. BARI baricitinib, BSA body surface area, EASI Eczema Area and Severity Index, EASI75 ≥ 75% improvement in Eczema Area and Severity Index, NRS numerical rating scale, N number, PBO placebo, TCS topical corticosteroid
A significantly greater proportion of patients receiving BARI 4-mg versus placebo achieved Itch NRS ≥ 4-point improvement response in the BSA 10–40%/Itch NRS ≥ 7 subgroup, with 58% of patients achieving this response at week 16 (vs. 22% on placebo). Similarly, 50% of patients with BSA 10–40%/Itch NRS < 7 showed an Itch NRS ≥ 4-point improvement response at week 16, yet it was not significantly different versus placebo (17%) (Fig. 3). This might be related to the small sample of patients with Itch NRS ≥ 4 at baseline in this subgroup. Patients with BSA > 40% and Itch < 7/ ≥ 7 showed response rates of 11% and 49%, respectively, at week 16, which were not statistically significantly different to placebo in both subgroups (Fig. 3C, D).
Itch ≥ 4-point improvement response rate over 16 weeks across identified BSA/Itch NRS subgroups. Proportion of patients achieving a ≥ 4-point improvement in Itch NRS with A BSA 10–40%/Itch NRS < 7, B BSA 10–40%/Itch NRS ≥ 7 (itch-dominant phenotype), C BSA > 40%/Itch NRS < 7, and D BSA > 40%/Itch NRS ≥ 7. Itch 4-point improvement was assessed in patients presented with Itch NRS ≥ 4 or at baseline; therefore, the n for subgroups (A, C) is reduced. p-values are based on Fisher’s exact test. *p < 0.05, **p < 0.01, and ***p < 0.001. BARI baricitinib, BSA body surface area, NRS Numerical Rating Scale, N number, PBO placebo, TCS topical corticosteroid
Among patients with ADSS Item 2 score of ≥ 1.5 at baseline, 82% and 83% of patients in the BSA 10–40%/Itch NRS ≥ 7 and BSA 10–40%/Itch NRS < 7 subgroups, respectively, achieved a 1.5-point improvement response in ADSS Item 2 at week 16 (Fig. 4), whereas 67% of patients with BSA > 40%/Itch < 7 and 62% patients with BSA > 40%/Itch NRS ≥ 7 achieved this response. None of these outcomes were significantly different versus placebo at week 16 for any of the subgroups.
Proportion of patients achieving ADSS ≥ 1.5-point improvement from baseline over 16 weeks for A BSA 10–40%/Itch NRS < 7, B BSA 10–40%/Itch NRS ≥ 7 (itch-dominant phenotype), C BSA > 40%/Itch NRS < 7, and D BSA > 40%/Itch NRS ≥ 7. p-values are based on Fisher’s exact test. *p < 0.05, **p < 0.01, and ***p < 0.001. ADSS Atopic Dermatitis Sleep Scale, BARI baricitinib, BSA body surface area, NRS Numerical Rating Scale, N number, PBO placebo, TCS topical corticosteroid
Discussion
In this study, we demonstrate that BSA involvement of 10–40% and high itch burden characterize adult AD patients that are likely to benefit most from treatment with BARI 4-mg. Using this clinical tailoring approach, about 70% of patients treated with BARI 4-mg in TCS combination therapy were able to reach an EASI75 response and about 60% a clinically relevant itch response after 16 weeks of treatment. Precision medicine is becoming increasingly important with the emergence of new therapies, especially in a highly heterogenous disease such as AD. The understanding of which patients are likely to benefit most from a specific treatment can help tailor therapies to individual patients and their specific needs. This approach can thereby improve the management of AD through enhancing patient experience, providing cost-effective therapies, and ensuring that only patients who are likely to benefit from a therapy receive it.
CART analysis, an unsupervised machine learning approach, consistently identified BSA involvement of about 40% as strongest baseline disease variable predicting response to BARI 4-mg in both monotherapy (BREEZE-AD1/2) and in combination with TCS (BREEZE-AD7), and across different response outcomes. This analysis also considered improvements on both signs (EASI75) and symptoms (Itch NRS 4-point improvement) of AD at week 16. These findings are in line with an earlier analysis identifying BSA as predictor for response to BARI 2-mg in the monotherapy trial BREEZE-AD5 [12]. Interestingly, although BSA was derived from EASI scoring, baseline EASI itself did not appear as a predicting factor for response at week 16 using CART. As expected, mean EASI was lower in those with a BSA of 10–40% compared to those with a BSA > 40%; however, a third of patients with itch-dominant AD had severe AD based on EASI (> 21) and reached EASI scores up to 30.5. In terms of mean SCORAD, patients with BSA 10–40% also ranged in the severe category (SCORAD > 50), especially those with high itch burden. The European treatment guideline recommends that these patients (SCORAD > 50) are candidates for systemic therapy [7]. While the prevalence of the identified subgroup of patients remains to be elucidated in a real-world setting, a significant proportion of patients who are candidates for systemic therapy in clinical practice might fall within this identified group of patients with AD. Indeed, data from real-world clinical practice suggest that patients with moderate-to-severe AD have generally lower BSA involvement as compared to patients enrolled in clinical trials and an affected mean BSA more in line with patients in the BSA 10–40% than in the BSA > 40% subgroup of the current analysis [13, 14].
This analysis focused on AD patients treated with BARI 4-mg in the TCS combination therapy trial BREEZE-AD7 to identify baseline characteristics predicting clinical response. BARI 4-mg is the primary recommended starting dose in Europe, Japan [15,16,17], and most other countries, and may be used in combination with TCS, which is reflective of routine AD clinical management [7]. Whether the clinical tailoring approach described in this study holds true in a real-world setting remains to be seen. Thus far, reports on experiences with BARI in clinical practice have not included information on baseline BSA [18,19,20,21]. However, a recent analysis which included 36 patients treated with BARI in Japan reported baseline itch levels similar to the itch-dominant subgroup in this study, with 64.7% of those patients reaching ≥ 4-point improvement in Itch NRS at week 12. Using correlation analysis, the authors proposed that a high EASI baseline score of the lower limbs was a predictor of response to BARI; however, how this relates to BSA in these patients has not yet been determined. Ongoing observational studies, such as AD-REAL (EUPAS37841) and atopic dermatitis registries, will provide critical data sources to confirm this concept in a real-world setting.
In the current post hoc analysis, patients treated with BARI 4-mg in combination with TCS showed significantly higher EASI75 response rates at week 16 compared to placebo with TCS in subgroups with BSA 10–40% at baseline. These EASI75 response rates were twice as high as in subgroups with BSA > 40% at baseline, in line with the predicted CART threshold, and reaching response levels of about 70% in patients with itch-dominant AD (BSA 10–40%/Itch NRS ≥ 7). These response rates are favorable compared to the ITT population (48% for BARI 4-mg) [7] and are comparable to those from other systemic therapies for the treatment of AD [22]. Similarly, clinically relevant itch response rates were enhanced, specifically in the itch-dominant subgroup (58%, with 50% in the BSA 10–40%/Itch NRS < 7 subgroup), compared to the ITT population (44%) when treated with BARI 4-mg, also reflected in an improvement of sleep disturbance due to itch. Enhanced treatment response in this subgroup of patients has also been confirmed when using BARI 4-mg in monotherapy, specifically when compared to subgroups with BSA > 40% (Supplementary Table 2). Higher response rates in patients with low BSA (10–40%) have also been reported previously for BARI 2-mg. Overall, these subgroup analyses confirm CART analyses and indicate that patients with BSA involvement of 10–40% and especially those with severe itch burden are likely to benefit most from BARI treatment.
The subgroup of AD patients identified to benefit most from BARI 4-mg seems identical to the unique “itch-dominant” AD phenotype described by Chovatiya et al. using lesional severity (EASI ≤ 21) and severe itch on a verbal rating scale, which accounted for a quarter (24%) of adult patients treated in clinical practice [4]. Although defined using lesion extent rather than lesion severity, the “itch-dominant” AD phenotypes identified in the current study and described previously [4] seem consistent in that they are characterized by high quality of life impairment, and considerable sleep and mental health disturbance, comparable to that of patients with high skin involvement (EASI > 21 and BSA > 40%, respectively), while having a low itch burden (Itch NRS < 7). This is in line with studies showing that itch has a significant impact on patients’ quality of life, sleep, and mental health [23, 24]. Despite this high burden, especially compared to patients with high skin involvement and low/moderate itch severity, patients with itch-dominant AD seem to be underappreciated, considering that they were reported to be less likely to be treated with systemic treatments compared to patients with high skin involvement. This was also reflected in a discrepancy between patient and physician-assessed perception of overall AD severity. [4]. Yet, due to the significant burden, these patients might be in need of rapid control of itch. BARI 4-mg has been demonstrated to improve itch as early as 2 days after initiating treatment [7], and more rapidly compared to dupilumab, based on an indirect comparison [22]. This underlines that patients with itch-dominant AD, as identified in this study, might indeed benefit most from BARI 4-mg treatment.
The limitations of this study are that it is a post hoc analysis limited to a 16-week placebo-controlled study period, and it includes low patient numbers in the subgroups analyzed. The safety profile of baricitinib has been well characterized in an integrated safety analysis involving 2636 patients with moderate-to-severe AD with exposure up to 3.9 years, and has been previously published [25].
Conclusion
Using a machine learning approach, we have defined the optimal profile for adult patients with moderate-to-severe AD who benefit most from BARI 4-mg treatment. These patients are characterized by BSA involvement between 10 and 40% with a high itch burden (Itch NRS > 7), and are most likely to show favorable response rates in improving AD signs and symptoms, specifically itch. In this subgroup of patients, 69% achieved an EASI75 and 58% an Itch NRS ≥ 4-point improvement response after 16 weeks of treatment. Providing patient profiling and characterization of patient populations likely to benefit most from BARI 4-mg treatment may allow precision medicine when making individual treatment decisions, and may ultimately enhance patient experiences, a concept that remains to be confirmed using real-world data sources.
References
Bieber T, et al. Clinical phenotypes and endophenotypes of atopic dermatitis: Where are we, and where should we go? J Allergy Clin Immunol. 2017;139(4s):S58-s64.
Renert-Yuval Y, et al. Biomarkers in atopic dermatitis-a review on behalf of the International Eczema Council. J Allergy Clin Immunol. 2021;147(4):1174-1190.e1.
Kantor R, et al. Research letter: impact of pruritus on quality of life—a systematic review. J Am Acad Dermatol. 2016;75(5):885-886.e4.
Chovatiya R, et al. Clinical phenotyping of atopic dermatitis using combined itch and lesional severity: a prospective observational study. Ann Allergy Asthma Immunol. 2021;127(1):83-90.e2.
Olumiant. European Union Summary of Product Characteristics. Eli Lilly and Company. 2021 [cited 2023 10 February]; https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-productinformation_en.pdf. Accessed 10 Feb 2023.
Wollenberg A, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–31.
Reich K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–43.
Cleophas TJZ, Aeilko H. Regression trees: classification and regression tree (CART) models. In: Regression analysis in medical research. 2021; Springer International Publishing, p. 383–391.
Terry Therneau [aut], et al. Recursive Partitioning and Regression Trees. 2022 27 January 2023]; https://cran.r-project.org/web/packages/rpart/rpart.pdf. Accessed 27 Jan 2023.
Simpson EL, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–55.
Vakharia PP, et al. Severity strata for five patient-reported outcomes in adults with atopic dermatitis. Br J Dermatol. 2018;178(4):925–30.
Silverberg JI, et al. Clinical tailoring of baricitinib 2 mg in atopic dermatitis: baseline body surface area and rapid onset of action identifies response at week 16. Dermatol Ther. 2022;12(1):137–48.
Thyssen JP, et al. Treatment of adult atopic dermatitis patients according to disease characteristics and demographics. Dermatol Ther. 2020;33(6): e14439.
Heratizadeh A, et al. Baseline characteristics, disease severity and treatment history of patients with atopic dermatitis included in the German AD Registry TREATgermany. J Eur Acad Dermatol Venereol. 2020;34(6):1263–72.
Radi G, et al. Baricitinib: the first Jak inhibitor approved in europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9(11):1575.
www.ema.europa.eu. Olumiant 2mg and 4mg film-coated tablets -Summary of Product Characteristics (SPC)-(eMC). 2020. 26 January 2023]; https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant#product-information-section. Accessed 26 Jan 2023.
Uchiyama A, et al. Real-world effectiveness and safety of baricitinib in Japanese patients with atopic dermatitis: a single-center retrospective study. J Dermatol. 2022;49(4):469–71.
Hagino T, et al., Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023. https://doi.org/10.1111/1346-8138.16763
Vittrup I, et al. Short-term real-world experience with baricitinib treatment in Danish adults with moderate-severe atopic dermatitis. J Eur Acad Dermatol Venereol. 2023;37(4):e543–6.
Boesjes CM, et al. Daily practice experience of baricitinib treatment for patients with difficult-to-treat atopic dermatitis: results from the BioDay Registry. Acta Derm Venereol. 2022;102:adv00820.
Rogner D, Biedermann T, Lauffer F. Treatment of Atopic Dermatitis with Baricitinib: First Real-life Experience. Acta Derm Venereol. 2022;102:adv00677.
de Bruin-Weller MS, et al. Indirect treatment comparison of baricitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis. Dermatol Ther (Heidelb). 2022;12(6):1481–91.
Hong J, et al. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30(2):71–86.
Pavlis J, Yosipovitch G. Management of itch in atopic dermatitis. Am J Clin Dermatol. 2018;19(3):319–32.
Bieber T, et al. Safety of baricitinib for the treatment of atopic dermatitis over a median of 1.6 years and up to 3.9 years of treatment: an updated integrated analysis of eight clinical trials. J Dermatolog Treat. 2023;34(1):2161812.
Acknowledgements
The authors would like to thank Dr Inmaculada de Torre MD, PhD of Eli Lilly and Company for her scientific input, contribution to the study design and analyses interpretation. We thank the participants of the study.
Funding
This work was supported by Eli Lilly and Company, which contributed to study design, data collection, data analysis, data interpretation, manuscript preparation, and publication decisions. Support for this assistance was funded by Eli Lilly and Company.
Medical Writing and Editorial Assistance
Medical writing assistance in the preparation of this article was provided by Joyce O’Grady, PhD, employee of Eli Lilly and Company.
Author Contributions
Susanne Grond, Christopher Schuster, and Maria Jose Rueda contributed to conception and design of the study. Yun-Fei Chen and Chunyuan Liu performed the statistical analysis. Susanne Grond, Christopher Schuster, Yun-Fei Chen, Chunyuan Liu, Maria Jose Rueda, Jacob P. Thyssen, Marjolein de Bruin-Weller, Antonio Costanzo, Andreas Pinter, and Thomas Bieber contributed to manuscript revision and read and approved the submitted version.
Disclosures
Susanne Grond, Chistopher Schuster, Yun-Fei Chen and Maria Jose Rueda are employees and minor shareholders of Eli Lilly and Company. Chunyuan Liu is a consultant from Tigermed-BDM Inc. working for Eli Lilly and Company. Jacob P. Thyssen an advisor for AbbVie, Almirall, Arena Pharmaceuticals, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, a speaker for AbbVie, Almirall, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme, and received research grants from Pfizer, Regeneron, and Sanofi-Genzyme. Marjolein de Bruin-Weller has been a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Arena, Aslan, Eli Lilly, Galderma, Janssen, LEO Pharma, Pfizer, Regeneron, and Sanofi-Genzyme. Antonio Costanzo served as advisory board member and consultant, and has received fees and speaker’s honoraria or has participated to clinical trials for Abbvie, Almirall, Biogen, Eli Lilly, LEO Pharma, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. Andreas Pinter worked as an investigator and/or speaker and/or advisor for the following pharmaceutical companies: AbbVie, Almirall-Hermal, Amgen, Biogen Idec, Biontec, Böehringer-Ingelheim, Celgene, GSK, Eli Lilly, Galderma, Hexal, Janssen, LEO-Pharma, MC2, Medac, Merck Serono, Mitsubishi, MSD, Norvartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi-Genzyme, Schering-Plough and USB Pharma. Thomas Bieber was speaker and/or consultant and/or Investigator for AbbVie, Allmiral, AnaptysBio, Arena, Asana Biosciences, Bayer Health, BioVerSys, Böhringer-Ingelheim, Bristol-Myers Squibb, Celgene, Daichi-Sankyo, Dermavant/Roivant, DermTreat, Domain Therapeutics, DS Pharma, Eli Lilly, Galapagos/MorphoSys, Galderma, Glenmark, GSK, Incyte, IQVIA, Janssen, Kirin, Kymab, LEO Pharma, LG Chem, L´Oréal, MenloTx, Novartis, OMPharma/Vifor, Pfizer, Pierre Fabre, RAPT/FLX Bio, Sanofi/Regeneron, UCB-Pharma. TB is founder of the non-profit biotech company “Davos Biosciences” within the International Kühne-Foundation.
Compliance with Ethics Guidelines
All studies were conducted in accordance with ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and the research protocols were approved by each centre’s institutional review board or ethics committee at each site. Details of the institutions and their ethics committees have been previously published [7, 10]. All patients provided written informed consent.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available upon request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at https://vivli.org/.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/
About this article
Cite this article
Thyssen, J.P., de Bruin-Weller, M., Costanzo, A. et al. Baseline Body Surface Area and Itch Severity Define Response to Baricitinib in Patients with Moderate-to-Severe Atopic Dermatitis at Week 16. Adv Ther 40, 3574–3587 (2023). https://doi.org/10.1007/s12325-023-02528-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02528-8