Abstract
Introduction
Preoperative ureteral catheterization/stenting (stenting) and intraoperative diagnostic cystoscopy (cystoscopy) may help prevent or identify intraoperative ureteral injuries (IUIs) during abdominopelvic surgery. In order to provide a comprehensive, single source of data for health care decision makers, this study aimed to catalog the incidence of IUI and rates of stenting and cystoscopy across a wide spectrum of abdominopelvic surgeries.
Methods
We conducted a retrospective cohort analysis of United States (US) hospital data (October 2015–December 2019). IUI rates and stenting/cystoscopy use were investigated for gastrointestinal, gynecological, and other abdominopelvic surgeries. IUI risk factors were identified using multivariable logistic regression.
Results
Among approximately 2.5 million included surgeries, IUIs occurred in 0.88% of gastrointestinal, 0.29% of gynecological, and 1.17% of other abdominopelvic surgeries. Aggregate rates varied by setting and for some surgery types were higher than previously reported, especially in certain higher-risk colorectal procedures. Prophylactic measures were generally employed at a relatively low frequency, with cystoscopy used in 1.8% of gynecological procedures and stenting used in 5.3% of gastrointestinal and 2.3% of other abdominopelvic surgeries. In multivariate analyses, stenting and cystoscopy use, but not surgical approach, were associated with a higher risk of IUI. Risk factors associated with stenting or cystoscopy, as well as those for IUI, largely mirrored the variables reported in the literature, including patient demographics (older age, non-White race, male sex, higher comorbidity), practice settings, and established IUI risk factors (diverticulitis, endometriosis).
Conclusion
Use of stenting and cystoscopy largely varied by surgery type, as did rates of IUI. The relatively low use of prophylactic measures suggests there may be an unmet need for a safe, convenient method of injury prophylaxis in abdominopelvic surgeries. Development of new tools, technology, and/or techniques is needed to help surgeons identify the ureter and avoid IUI and the resulting complications.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Intraoperative ureteral injury (IUI) is a serious surgical complication that can arise in the setting of abdominopelvic surgeries. Currently available data are either exclusively from single-center studies or specific to certain procedures. Thus, there is a need for a compendium of data to help health care decision makers benchmark the rate of IUI and prophylactic procedures at their institution. |
Our objective was to compile the incidence of IUI in abdominopelvic surgical procedures, estimate the rates of stenting and cystoscopy used to prevent or diagnose IUI in such settings, and assess factors that may contribute to IUI and/or the decision to prophylax against injury. |
What was learned from the study? |
Use of stenting and cystoscopy largely varied by surgery type, as did rates of IUI. IUI rates were found, in select instances, to be higher than rates previously reported. |
Stenting appeared to be associated with IUI, but future prospective studies are needed to discern whether it is, indeed, a true risk factor or the confirmation that stents are more likely to be employed in surgeries associated with the highest risk of IUI. |
Results suggest there may be an unmet need for a safe, convenient method of injury prophylaxis in abdominopelvic surgeries, as well as new tools, technology, or techniques to help surgeons identify the ureter and avoid IUI and the resulting complications. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.22551226.
Introduction
Patient safety is the health care executive’s foremost concern [1]. With accreditation, reimbursement, and community standing all tied to patient safety metrics, developing successful, sustainable, procedure-based service lines relies heavily upon delivery of care with a low complication rate [1, 2]. To that end, intraoperative ureteral injury (IUI) is a serious surgical complication that can arise in the setting of abdominopelvic surgeries. The retroperitoneal position of the ureter near or posterior to other abdominal and pelvic structures may compromise its visualization during surgery and increase the risk of accidental ligation, laceration, mechanical damage, thermal injury, or devascularization [3]. Serious complications from IUI can include ureteral obstruction or fistula, abscesses, sepsis, renal failure, and death [3, 4].
Prevention of IUI is important to surgeons because of the potential for serious complications [5]. Although IUIs can increase length of stay and hospital costs [6], intraoperative recognition of an IUI may limit complications [3, 7, 8]. Unfortunately, many IUIs are identified postoperatively [8]. Preoperative or intraoperative ureteral catheterization (hereafter referred to as stenting), performed in almost all instances by cystoscopy, can be used to visualize the ureter and identify incident injuries during surgery, but the practice is not routinely recommended in surgical guidelines [3, 8, 9]. In some settings of suspected injury, such as minimally invasive (laparoscopic) hysterectomies, intraoperative diagnostic cystoscopy (hereafter referred to as cystoscopy) may be used to diagnose an IUI during surgery [7]. However, the evidence regarding the prophylactic effectiveness of stenting in preventing and identifying IUI, and of cystoscopy in identifying IUI in hysterectomies, remains inconclusive [8, 10].
Published data describing the incidence of IUIs during abdominopelvic surgeries and the use of stents or cystoscopy over the last several years are limited. Often, studies focus on a specific type of high-volume surgery, primarily hysterectomy and colectomy [5, 11,12,13,14,15,16,17,18]. Information on stent use and cystoscopy is particularly sparse. Lacking most is a single-source compendium, derived from a national data set, that can be accessed by service line managers and health care executives to help benchmark the rate of ureteral injury and prophylactic procedures at their institution. Ideally, such a resource would inform against the entire spectrum of abdominopelvic procedures and not just those which are most commonly performed. This study, therefore, had three distinct aims: (1) to compile the incidence of IUI across a diverse array of gastrointestinal (GI), gynecological (GYN), and other abdominopelvic surgical procedures; (2) to estimate the rates of stenting and cystoscopy used to prevent or diagnose IUI in such settings; and (3) to assess the factors which may contribute to IUI and/or the decision to prophylax against injury. The availability of these data in a single-source document may lead to additional patient safety measures being adopted in operating rooms, where applicable.
Methods
Data Source
This retrospective cohort study used data from the Premier Healthcare Database, a large, hospital-based, service-level, all-payer database that contains data from over 271 million unique patients from geographically diverse hospitals and health systems in the USA [19]. It includes more than 8.5 million annual inpatient admissions, representing approximately 25% of annual US inpatient admissions, and more than 125 million annual outpatient encounters to emergency departments, ambulatory surgery centers, and alternate sites of care. It contains information on hospital and visit characteristics, physician specialties, patient data (e.g., demographics, diagnoses), and billed services (e.g., medications, devices). Given the retrospective nature of this study, institutional review board approval was not deemed necessary. In accordance with the HIPAA Privacy Rule, disclosed Premier Healthcare Database data are considered deidentified per 45 CFR 164.506(d)(2)(ii)(B) through the “Expert Determination” method. This study was conducted in compliance with national requirements for noninterventional studies using deidentified data. Analysis of Premier Healthcare Database data was permitted per the data licensing agreement [19].
Study Population
The study evaluated abdominopelvic surgical procedures in which the ureter would typically be encountered within the operative field—those in which an IUI would be most likely to occur (Supplementary Table S1). On the basis of clinical expertise, the authors selected surgeries according to the degree to which a surgeon would expect to contend with retroperitoneal structures intraoperatively or whether there would be a need for organ mobilization from the retroperitoneum.
Surgeries in adult patients (aged ≥ 18 years at the surgery date) between October 2015 and December 2019 were included. Surgeries were primarily identified and categorized using the International Classification of Diseases 10th revision (ICD-10) procedure coding system (PCS). Current Procedural Terminology (CPT) codes were used when ICD-10-PCS codes were not available (see Supplementary Methods).
In order to distinguish ureteral procedures used for surgical prophylaxis from those used to treat a primary urologic condition, index surgeries were excluded from the analysis if certain prespecified elective urological surgeries (e.g., kidney calculous removal) or conditions (e.g., ureteral stone) were also performed on the index date. Surgeries with important missing or invalid data or values, including potentially duplicated records, were also excluded. Patients were followed from the surgery date (index date) for up to 4 months.
Study Outcomes
The study outcomes included rates of IUI, stenting, and cystoscopy use in the index surgery. IUIs were identified by ICD-10 diagnosis code (S37.1x) or procedure codes [5, 6, 11,12,13, 17, 18, 20,21,22].
Stenting and cystoscopy use were defined by CPT code, billing code, or ICD-10-PCS codes on the day of surgery (Supplementary Table S2) [5, 6, 11, 22]. Cystoscopy use was investigated only in GYN surgeries. In cases where both stenting and cystoscopy were listed, only the most invasive procedure (stenting) was captured because it was assumed that cystoscopy would be required to place all prophylactic ureteral catheters.
Statistical Analysis
The IUI rate and stenting and cystoscopy rates were reported with 95% CIs. Inpatient surgery projection weights contained in the Premier Healthcare Database were used to estimate standardized stenting, cystoscopy, and IUI rates by US geographic region/division for inpatient surgeries. For outpatient surgeries, projection weights were created as the ratio between projected population outpatient surgery counts and outpatient surgery counts observed in the database. Logistic regression analyses were conducted to assess risk factors. Baseline characteristics included in the model and their specifications were selected according to clinical and statistical importance (Supplementary Table S3). No interaction terms were included. More details on statistical analyses are provided in the Supplementary Methods.
Results
Baseline Characteristics
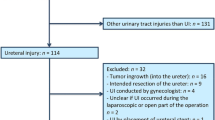
In total, 2,498,458 surgeries were included in the analysis (Fig. 1). GYN surgeries accounted for the largest portion of the study cohort (Table 1). Approximately half of GI and other abdominopelvic surgeries were minimally invasive surgeries (56.28% and 53.78%, respectively), whereas most GYN surgeries were open surgeries (65.50%).
Most GI surgeries were performed as inpatient procedures and most were elective; 249,395 procedures were performed on the vermiform appendix. Among GYN surgeries, procedures were generally performed in the outpatient setting, except for cesarean sections (1,020,451/1,788,478; 57.06%), which were performed in the inpatient setting. GYN surgeries were rarely performed as emergency procedures. Peritoneal cavity procedures accounted for 46.13% of the other abdominopelvic surgeries; settings and admission type largely varied in this category.
IUI Incidence
Overall, 11,648 IUIs were identified (0.46%), 30.5% of which occurred in inpatient elective GI surgeries, 28.5% in outpatient elective GYN surgeries, 16.0% in inpatient emergency GI surgeries, and 13.7% in inpatient elective GYN surgeries. The IUI rate was 0.88% in GI surgeries, 0.29% in GYN surgeries, and 1.17% in other abdominopelvic surgeries (Supplementary Table S4). When excluding appendix procedures and cesarean sections (sensitivity analysis), the injury rate was 0.83% (10,189/1,228,612) in total, 1.31% (5261/402,375) in GI surgeries, and 0.64% (4928/768,027) in GYN surgeries. By setting, 30.46% (3548/11,648) of IUIs occurred during inpatient elective GI surgeries, 28.45% (3314/11,648) during outpatient elective GYN surgeries, 16.00% (1864/11,648) during inpatient emergency GI surgeries, and 13.73% (1599/11,648) during inpatient elective GYN surgeries.
IUI Incidence in GI Surgeries
By setting, IUI incidence for GI surgeries excluding appendiceal procedures was highest for inpatient elective procedures (1.41%, 95% CI 1.37–1.46; n/N = 3548/251,482) (Fig. 2). In the inpatient elective setting, the highest IUI incidence was observed in colostomy cases (3.04%, 95% CI 2.67–3.44; 245/8065), followed by enterostomy (2.71%, 95% CI 2.32–3.15; 167/6164); proctectomy, partial proctectomy, or total proctocolectomy (all with anastomosis) (2.66%, 95% CI 2.30–3.07; n/N = 184/6911); and removal of rectal segment without anastomosis (2.24%, 95% CI 1.89–2.65; n/N = 138/6151). Specifically for colectomies, IUI incidence was 1.34% (95% CI 1.28–1.40; n/N = 1895/141,321) overall. Appendectomies comprised 38.17% of GI procedures (n/N = 248,809/651,770) and had an IUI incidence of 0.19% (n/N = 476/248,809). If appendectomies are excluded, the overall IUI rate in GI surgeries increases by a factor 1.5 to 1.31% (n/N = 5266/402,961).
IUI Incidence in GYN Surgeries
By setting, the highest IUI incidence for GYN surgeries minus cesarean sections was for outpatient elective procedures (0.60%, 95% CI 0.58–0.62; n/N = 3314/551,131), although the highest IUI incidence was observed in inpatient emergency cervical procedures (4.75%, 95% CI 3.68–6.03; n/N = 64/1347). Specifically for hysterectomies, IUI incidence was 0.80% (n/N = 2907/363,643) overall. A similar IUI incidence was observed between inpatient (0.86%, 95% CI 0.80–0.91; n/N = 939/109,438) and outpatient (0.76%, 95% CI 0.73–0.79; n/N = 1884/248,293) elective hysterectomies, and inpatient (1.45%, 95% CI 1.13–1.83; n/N = 69/4759) and outpatient (1.30%, 95% CI 0.73–2.14; n/N = 15/1153) emergency hysterectomies.
IUI Incidence in Other Abdominopelvic Surgeries
By setting, the highest IUI incidence was for inpatient elective procedures (1.64%, 95% CI 1.47–1.83; n/N 340/20,686). In the inpatient setting, IUI incidence was generally higher in elective compared to emergency surgeries (Fig. 2). The exception was for retroperitoneal procedures, where the highest incidence was observed in inpatient emergency compared with elective surgeries (3.82% [n/N = 40/1046] vs 3.44% [n/N = 82/2382], respectively).
Preoperative Ureteral Catheterization or Stenting Use
Across all surgeries, stenting was seldom used (2.32% of GI surgeries, 0.19% of GYN surgeries, 0.95% of other abdominopelvic surgeries; Fig. 3). Stenting was generally used more often in the inpatient elective setting (Supplementary Table S5). Rates of stenting by individual procedure were generally low, as described in the following sections.
Stenting Use in GI Surgeries
In inpatient elective GI surgeries, stenting use was most frequently observed in elective proctectomies with anastomosis (9.82%), enterostomy (i.e., bypass of small intestine to the skin; 9.57%), removal of rectal segment without anastomosis (7.77%), removal of colon segment without anastomosis (7.70%), and subtotal or partial (segmental) colectomy with re-anastomosis (6.31%). For elective proctectomies with anastomosis, total proctocolectomy (removal of entire colon together with rectum) may or may not be included in this category. If the proctectomy was selected as the primary procedure by the hospital, the surgery is categorized in this group. In cases where the primary procedure was colectomy, the surgery was counted in the colectomy category. In inpatient emergency GI surgeries, stenting was most frequently used in emergency bypass/resection of rectum (5.42%) and excision of rectum (3.70%) (Fig. 4). Specifically for colectomies, stenting was used in 5.05% (n/N = 7131/141,321) of these surgeries overall.
Stenting Use in GYN Surgeries
In inpatient elective GYN surgeries, stenting use was most frequently observed in procedures on uterine supporting structures (4.02%), paravaginal procedures (2.77%), cul-de-sac procedures (2.37%), oophorectomy (2.35%), and ovariolysis and/or salpingolysis (2.04%) surgeries (Fig. 5). In the emergency setting, stenting was observed most frequently in uterine supportive structure procedures (1.96%), oophorectomy (1.78%), and hysterectomy (1.70%). Specifically for hysterectomies, stenting was used in 0.57% (n/N = 2071/363,643) of these surgeries overall.
Stenting Use in Other Abdominopelvic Surgeries
In inpatient elective and emergency other abdominopelvic surgeries, stenting use was most frequently observed for retroperitoneal procedures (elective, 7.60%; emergency, 1.82%) (Supplementary Fig. S1). Stenting was seldom used in the outpatient setting.
Intraoperative Diagnostic Cystoscopy Use in GYN Surgeries
Cystoscopy was used in 1.83% of GYN surgeries (Fig. 6), but only in 0.1% of cesarean sections, which constituted 57.1% of the GYN procedures in this analysis. In the inpatient setting, cystoscopy was used in 1.60% of elective procedures and 1.07% of emergency procedures. In the outpatient setting, it was used in 2.5% of elective surgeries and 0.56% of emergency surgeries.
By procedure, high cystoscopy use was observed in inpatient elective paravaginal procedures (30.50%) and cul-de-sac procedures (30.32%) (Supplementary Fig. S2). In the inpatient emergency setting, cystoscopy use was observed most in hysterectomy (12.01%) and paravaginal procedures (11.66%). In the outpatient setting, high cystoscopy use was observed in elective procedures on uterine supporting structures (7.56%), cul-de-sac procedures (7.15%), and vaginal procedures (6.00%). Specifically for hysterectomies, cystoscopy was used in 6.84% of these procedures overall.
US Geography
Stenting rates were generally higher in geographical areas with higher IUI rates (Supplementary Fig. S3). For outpatient GYN surgeries, stenting and IUI rates were highest in West North Central states and cystoscopy rates were highest in Mountain states (Supplementary Fig. S3a). For inpatient surgeries, the highest IUI rate was observed in other abdominopelvic surgeries in the East North Central region (2.1%) and the highest stenting rate was in GI surgeries in the Middle Atlantic region (8.42%) (Supplementary Fig. S3b). Rates of cystoscopy use and IUI were both relatively low in inpatient GYN surgeries across geographic regions (range 1.18–3.11% and 0.12–0.21%, respectively).
Factors Associated with IUI
Prespecified risk factors for IUI were explored using patient and surgery characteristics (Fig. 7). The full listing of results is provided in Supplementary Tables S6–S9. Stenting, cystoscopy, older age (vs 18–34 years of age), male sex (except in GYN surgeries), and high comorbidity index were each associated with a higher risk of IUI in multivariable analyses for all abdominopelvic surgeries (overall and in GI, GYN, and other abdominopelvic surgeries). All prespecified risk factors were associated with higher IUI risk overall (Supplementary Table S6) and higher IUI risk associated with GI surgeries (Supplementary Table S7). Surgical approach was not associated with an increased risk of IUI in the total surgery population or other abdominopelvic surgeries.
In GI surgeries, in addition to the common risk factors mentioned previously, non-White race, elective surgeries, open-approach procedures, and surgeries at hospitals performing a high volume of GI cases were associated with an increased risk of IUI (Supplementary Table S7). In GYN surgeries, in addition to the common risk factors mentioned previously, Black race, emergency surgeries, oophorectomy and/or salpingectomy, procedures on uterine supportive structures, cervical procedures, vaginal procedures, cul-de-sac procedures (vs hysterectomy), and surgeons performing a low volume of GYN procedures (< 1 surgery/month) were associated with an increased risk of IUI (Supplementary Table S8). In other abdominopelvic surgeries, in addition to the common risk factors mentioned previously, non-White race, retroperitoneal procedures (vs peritoneal procedures), history of surgery, history of radiation, and perforation were associated with higher risk of IUI (Supplementary Table S9).
Discussion
In the current study, overall IUI rates for abdominopelvic surgeries were low. An incidence rate of 1% can be substantiated in GI and GYN procedures with the exclusion of appendiceal procedures and caesarean sections. Although this might seem like an acceptable or manageable level of risk, it is important to note that at this rate, most large tertiary care centers in the USA could expect to amass their own cohort of such injuries in a relatively short period of time. For example, if a large hospital system does 20,000 abdominopelvic surgeries per year across a number of facilities, a 1% injury rate represents 200 cases of injury each year. In addition, the IUI rate observed in this study is greater than the risk of pulmonary embolism in the hospitalized Medicare population (0.68%) [23]. Even more noteworthy is that for some cases (colostomy, ileostomy, proctectomy, and retroperitoneal procedures), higher rates (3–5%) were observed in our study; the high rate observed for colostomy cases was surprising. For context, the rate of catheter-associated urinary tract infections in hospitals for 2020 was reported at around 7.5% [24]. As the occurrence of IUIs can be catastrophic for the patient, it remains incumbent upon surgeons to be vigilant. From a health systems perspective, IUIs increase costs and are time-consuming and problematic to resolve [13, 25]. However, current IUI prevention methods may be burdensome, unproven, and inconvenient for surgeons and hospitals, resulting in low utilization, as we have observed. Accordingly, the field awaits the development of a convenient, cost-effective method of prevention. For example, the technique of real-time, fluorescence-guided imaging has shown promise in laparoscopic colorectal surgeries [26] and may emerge as a future option to help visualize the ureters.
With regard to stenting, rates were consistent with those previously reported for select surgeries. For example, in GI surgeries, the stenting rate in colectomy (5.05%) and colorectal (6.75%) surgeries was comparable to 4.9% of colectomies over the period from 2012 to 2014 and 4.2% of laparoscopic colorectal surgeries during the years 2005 to 2011 [5, 16]. For hysterectomies, the rates of stenting (0.6%) and cystoscopy (6.8%) use are lower than previously reported (1.4% and 16.3–25%, respectively) [11, 17]. By including cesarean sections (57.1% of GYN procedures), we artificially dampen the overall stenting and cystoscopy rate for GYN surgeries, because stenting and cystoscopy use were minimal in these procedures.
Across all surgeries evaluated in the study data set, surgical approach was not associated with an increased risk of IUI. However, consistent with other studies identified through a systematic review [27], stenting or cystoscopy use was associated with an increased risk of IUI in the present study population. A pooled analysis of the usefulness of prophylactic stenting for colorectal surgeries found higher IUI rates in a stented group (1.49%) compared to a no-stent group (0.17%) [27]. However, the authors did not perform a meta-analysis because of heterogeneity and low study quality of the referenced studies. Because higher IUI rates in stented patients could reflect greater stent use in higher-risk surgeries, the authors could not conclude whether stents increased ureteral injury and/or increased IUI detection [27]. Our study was also unable to tease apart this relationship, but future cohort studies might consider prospectively documenting the reason for stenting so that this can be further elucidated.
Although rates of IUI, stenting, and cystoscopy varied by surgery type and setting, when taken together in the multivariate risk factor analysis, stenting and cystoscopy were each associated with a higher risk of IUI. The reasons for this are unclear. Because the motive for stenting was not available from the Premier Healthcare Database (as is the case with many database studies), it is important to consider whether this association was due to reverse causation, a phenomenon where, in this case, an association might be observed between stenting and injury, but only because stents were likely being used as a means of treatment once an injury had occurred. Unfortunately, the nature of the data set precludes the ability to distinguish such cases from those in which a stent was placed prophylactically prior to surgery based on a perceived risk of injury. It should also be noted that the current study design is unable to account for a natural form of channeling bias, observed in those cases where stents are likely deployed in complicated clinical settings with a greater baseline chance of injury. Similarly, the motive for cystoscopy during GYN surgery was not available from the database. Although cystoscopy to visualize continuity of urine flow through the ureteral orifices is used by GYN surgeons to rule out injury, as a result of the nature of the data set, we cannot confirm that all documented cystoscopies were, in fact, used to interrogate possible ureteral injury.
With regard to practice setting, US regions with higher use of stenting also tended to have higher IUI rates (stenting use was highest in the North Central states, which also showed the highest IUI rates). Overall, stenting rates were still quite low and there was not much separation by region. Conversely, rates of cystoscopy were higher in outpatient elective GYN procedures, where no trends were observed between cystoscopy use and IUI rates. There was also no significant regional variation in cystoscopy use.
The majority of risk factors we identified are consistent with the literature on IUI. Older age and high comorbidity index were each associated with an increased risk of IUI overall and in the IUI risk for all abdominopelvic surgery categories. Male sex (overall, GI, other abdominopelvic surgeries) and Black (GYN surgeries) or non-White (GI, other abdominopelvic surgeries) race also increased risk of injury as did an assortment of certain risk factors (history of surgery within 6 months, history of radiation within 6 months, malignancy, endometriosis, diverticulitis, perforation of intestine, adhesions, obesity). Stenting was associated with an increased risk of IUI overall in all abdominopelvic surgery categories, while cystoscopy was also associated with increased IUI risk in GYN surgeries. Elective surgeries, open-approach procedures, and surgeries at hospitals performing a high volume of GI surgeries were associated with an increased risk of IUI in GI surgeries, a somewhat counterintuitive finding, but one that is likely explained by more complicated cases being referred to tertiary centers (another example of channeling bias). Emergency surgeries, oophorectomy and/or salpingectomy, procedures on uterine supportive structures, cervical procedures, vaginal procedures, cul-de-sac procedures (vs hysterectomy), and surgeons performing a low volume of GYN procedures (< 1 surgery/month) were associated with an increased risk of IUI in GYN surgeries, whereas retroperitoneal procedures (vs peritoneal procedures) were associated with higher risk in other abdominopelvic surgeries. In general, we found that the IUI risk factors identified in this study were consistent with the literature [5, 14, 15].
Despite their continued availability, stent use has remained relatively low over time, as results from this study and previous reports indicate [5, 16, 17], suggesting stenting in abdominopelvic surgeries is not widely accepted even for high-risk surgeries. There is also a perception, not refuted by this study or the aforementioned meta-analysis by Teeluckdharry et al., that stenting does not seem to lower the incidence of IUIs, but merely helps to identify them once they occur—although for some surgeons, ureteral stenting may provide a false sense of security [26]. Indeed, stent deployment may give a false sense of security particularly in low-risk situations, with the end result being delayed recognition of injury. Moreover, stenting for IUI prevention increases surgery and operating room time, often requiring the assistance of a urologist, which is costly for payers and resource intensive for hospitals. Finally, there are potential complications associated with stenting, such as hematuria, urinary tract infection, renal impairment, and irritative bladder symptoms, as well as a risk of ureteral injury during stent placement itself [27]. Although rates of stenting complications are low, they are important to consider given the potential impact to patients and hospital quality measures. One of the major potential self-fulfilling prophecies in these results is that stents may be selectively used in surgeries deemed to be at highest risk for IUI. This rationale would indeed explain the association between stents and IUI.
In addition to those already mentioned, our study has several limitations inherent in administrative database analyses. As a result of the limited use of the stenting CPT code (52005) in the data set (4.4% of procedures with stenting), this analysis used an array of ICD-10 codes to identify stent use, whereas prior studies have typically used CPT codes [5, 15,16,17,18]. Even so, some of the selected procedure and diagnosis codes used to define IUI in our study may not have been sensitive or specific enough for IUI or intraoperative injuries, potentially causing an error of estimating IUI incidence. This might explain the higher injury rates we observed in this study compared with previous studies [5, 15, 18]. Furthermore, the codes used to define GI and other abdominopelvic procedures in Premier both included codes for proctectomies. While we opted to include these cases under the GI category, they could have also been categorized as “other” according to the Premier nomenclature. Finally, because of the nature of the Premier Database itself, the results may not be representative of populations treated in other care settings.
Nevertheless, this study analyzed nearly 2.5 million surgeries among 847 US hospitals and examined IUI, stenting, and diagnostic cystoscopy rates in granular detail, thus opening a window on specific surgery types and practice settings. Its utility is grounded in its ability to serve as a surgical safety and operating room committee resource, a health systems reference, and a guide to third-party payers and reimbursement authorities. In this regard, its scope extends much further than those studies that are typically found in the literature, which tend to focus on aggregate findings and/or high-volume procedures while often featuring smaller populations [5, 16, 18]. Furthermore, our unique coding rubric allowed us to explore a much wider range of procedures to capture the extent of injury. Finally, results from our multivariable logistic regression models can be used to identify those patients most likely to undergo prophylactic procedures in the name of injury prevention as well as construct profiles for patients at risk of IUI during abdominopelvic surgeries.
Conclusions
Results from this large database analysis found that use of stenting and cystoscopy largely varied by surgery type, as did rates of IUI. Injury rates, however, were a function of setting/surgery type and were found, in select instances, to be higher than rates previously reported. Prophylactic measures were generally employed at a low frequency, suggesting there may be an unmet need for a safe, convenient method of injury prophylaxis. Risk factors associated with stenting or diagnostic cystoscopy, as well as those for IUI, largely mirrored the variables reported in the literature, including patient demographics, practice settings, and established IUI risk factors. Stenting appeared to be associated with IUI, which may be attributable to the use of stents during surgeries where the risk of IUI is thought to be highest. Future prospective studies will need to discern whether it is, indeed, a true risk factor. In the meantime, deployment of new technology is something the field anxiously awaits.
References
American College of Healthcare Executives. The healthcare executive's role in ensuring quality and patient safety. 2017. https://www.ache.org/about-ache/our-story/our-commitments/policy-statements/healthcare-executives-role-in-ensuring-quality-and-safety. Accessed 8 Aug 2022.
Modern Healthcare Insights. Hospital service line organization: Innovation in approaches and strategy. 2012. https://www.modernhealthcare.com/assets/pdf/CH81353810.PDF. Accessed 8 Aug 2022.
Engelsgjerd J, LaGrange C. Ureteral Injury. [Updated 2021 Jul 10]. Treasure Island (FL): StatPearls; 2021 Jan. https://www.ncbi.nlm.nih.gov/books/NBK507817/?report=classic.
Abboudi H, Ahmed K, Royle J, et al. Ureteric injury: a challenging condition to diagnose and manage. Nat Rev Urol. 2013;10:108–15.
Coakley KM, Kasten KR, Sims SM, et al. Prophylactic ureteral catheters for colectomy: a National Surgical Quality Improvement Program-based analysis. Dis Colon Rectum. 2018;61:84–8.
Halabi WJ, Jafari MD, Nguyen VQ, et al. Ureteral injuries in colorectal surgery: an analysis of trends, outcomes, and risk factors over a 10-year period in the United States. Dis Colon Rectum. 2014;57:179–86.
AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL Practice Report: Practice guidelines for intraoperative cystoscopy in laparoscopic hysterectomy. J Minim Invasive Gynecol. 2012;19:407–11.
Burks FN, Santucci RA. Management of iatrogenic ureteral injury. Ther Adv Urol. 2014;6:115–24.
Hassinger TE, Mehaffey JH, Mullen MG, et al. Ureteral stents increase risk of postoperative acute kidney injury following colorectal surgery. Surg Endosc. 2018;32:3342–8.
Teeluckdharry B, Gilmour D, Flowerdew G. Urinary tract injury at benign gynecologic surgery and the role of cystoscopy: a systematic review and meta-analysis. Obstet Gynecol. 2015;126:1161–9.
Barber EL, Polan RM, Strohl AE, Siedhoff MT, Clarke-Pearson DL. Cystoscopy at the time of hysterectomy for benign indications and delayed lower genitourinary tract injury. Obstet Gynecol. 2019;133:888–95.
Benson CR, Thompson S, Li G, Asafu-Adjei D, Brandes SB. Bladder and ureteral injuries during benign hysterectomy: an observational cohort analysis in New York State. World J Urol. 2020;38:2049–54.
Blackwell RH, Kirshenbaum EJ, Shah AS, et al. Complications of recognized and unrecognized iatrogenic ureteral injury at time of hysterectomy: a population based analysis. J Urol. 2018;199:1540–5.
Bretschneider CE, Casas-Puig V, Sheyn D, Hijaz A, Ferrando CA. Delayed recognition of lower urinary tract injuries following hysterectomy for benign indications: a NSQIP-based study. Am J Obstet Gynecol. 2019;221:132.e1–132.e13.
Packiam VT, Cohen AJ, Pariser JJ, et al. The impact of minimally invasive surgery on major iatrogenic ureteral injury and subsequent ureteral repair during hysterectomy: a national analysis of risk factors and outcomes. Urology. 2016;98:183–8.
Speicher PJ, Goldsmith ZG, Nussbaum DP, et al. Ureteral stenting in laparoscopic colorectal surgery. J Surg Res. 2014;190:98–103.
Wallis CJ, Cheung DC, Garbens A, et al. Occurrence of and risk factors for urological intervention during benign hysterectomy: analysis of the national surgical quality improvement program database. Urology. 2016;97:66–72.
Zafar SN, Ahaghotu CA, Libuit L, et al. Ureteral injury after laparoscopic versus open colectomy. JSLS. 2014;18:e2014.00158.
PINC AI™ Applied Sciences PI. PINC AI™ Healthcare Data: Data that informs and performs (White Paper). March 2022. 2022. https://offers.premierinc.com/rs/381-NBB-525/images/Premier-Healthcare-Database-Whitepaper-Final.pdf. Accessed 14 Oct 2022.
Eswara JR, Raup VT, Potretzke AM, Hunt SR, Brandes SB. Outcomes of iatrogenic genitourinary injuries during colorectal surgery. Urology. 2015;86:1228–33.
Frankman EA, Wang L, Bunker CH, Lowder JL. Lower urinary tract injury in women in the United States, 1979–2006. Am J Obstet Gynecol. 2010;202(495):e1–5.
Schimpf MO, Gottenger EE, Wagner JR. Universal ureteral stent placement at hysterectomy to identify ureteral injury: a decision analysis. BJOG. 2008;115:1151–8.
Minges KE, Bikdeli B, Wang Y, et al. National trends in pulmonary embolism hospitalization rates and outcomes for adults aged ≥65 years in the United States (1999 to 2010). Am J Cardiol. 2015;116:1436–42.
US Centers for Disease Control and Prevention. 2020 National and State Healthcare-Associated Infections Progress Report. 2021. https://www.cdc.gov/hai/data/portal/progress-report.html. Accessed 26 Oct 2022.
Locke JA, Matta R, Saskin R, et al. Impact of delayed recognition of iatrogenic ureteral injury in a retrospective population-based study. Urol Pract. 2021;8:636–44.
Satish VNVR, Acharya A, Ramachandran S, Narasimhan M, Ardhanari R. Fluorescent ureterography with indocyanine green in laparoscopic colorectal surgery: a safe method to prevent intraoperative ureteric injury. J Minim Access Surg. 2022;18:320–3.
Croghan SM, Zaborowski A, Mohan HM, et al. The sentinel stent? A systematic review of the role of prophylactic ureteric stenting prior to colorectal resections. Int J Colorectal Dis. 2019;34:1161–78.
Acknowledgements
Funding
This study was funded by Astellas Pharma, Inc. The sponsor participated in the study design, collection, analysis, and interpretation of data, writing of the report, and in the decision to submit the article for publication, as detailed in Author Contributions. The study sponsor also provided funding for the journal’s Rapid Service and Open Access fees.
Medical Writing, Editorial, and Other Assistance
Medical writing/editorial support was provided by Beth A. Lesher, PharmD, BCPS, and Catherine Mirvis, BA, from OPEN Health (Bethesda, MD), and Cheryl Casterline, MA, from Peloton Advantage, LLC, an OPEN Health company (Parsippany, NJ), and funded by the study sponsor, Astellas Pharma, Inc.
Author Contributions
Ana Filipa Alexandre, Tomomi Kimura, and Jason Schwartz contributed to the study conception and design; Tomomi Kimura, Qi Feng, and Wei Han acquired the data; Steven McCarus, Ana Filipa Alexandre, Tomomi Kimura, Qi Feng, Wei Han, Emily F. Shortridge, Robson Barbosa Lima, Jason Schwartz, and Steven D. Wexner analyzed and interpreted the data, drafted and critically revised the manuscript, and approved the final version.
Prior Presentation
A portion of the submitted work was presented during the 2021 American College of Surgeons Clinical Congress (virtual, October 23−27, 2021, #5759).
Disclosures
Steven McCarus reports consulting fees from Astellas; Tomomi Kimura, Qi Feng, Wei Han, Emily F. Shortridge, and Jason Schwartz are employees of Astellas Pharma, Inc. Ana Filipa Alexandre is a former employee of Astellas Pharma, Inc and was at the time of the study (current affiliation: Santen SA, the Netherlands). Robson Barbosa Lima is a former employee of Astellas Pharma, Inc. (current affiliation: AbbVie Inc.). Steven D. Wexner reports consulting fees from ARC, Astellas, Baxter, ICON, Stryker, Intuitive, Olympus, Medtronic; royalties for Intellectual Property licenses from Intuitive, Medtronic, and Karl Storz.
Compliance with Ethics Guidelines
Given the retrospective nature of this study, institutional review board approval was not deemed necessary. In accordance with the HIPAA Privacy Rule, disclosed Premier Healthcare Database data are considered deidentified per 45 CFR 164.506(d)(2)(ii)(B) through the “Expert Determination” method. This study was conducted in compliance with national requirements for noninterventional studies using deidentified data. Analysis of Premier Healthcare Database data was permitted per the data licensing agreement.
Data Availability
Researchers may request access to anonymized participant level data, trial level data and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx
Author information
Authors and Affiliations
Corresponding author
Additional information
Ana Filipa Alexandre was an Astellas employee at the time of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
McCarus, S., Alexandre, A.F., Kimura, T. et al. Abdominopelvic Surgery: Intraoperative Ureteral Injury and Prophylaxis in the United States, 2015–2019. Adv Ther 40, 3169–3185 (2023). https://doi.org/10.1007/s12325-023-02515-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02515-z