Abstract
Introduction
First-line treatment with toripalimab plus paclitaxel and cisplatin (TTP) is very effective for patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC) in China, although its effects on economic burden are unknown. The present study aimed to evaluate the cost-effectiveness of TTP from the perspective of the Chinese healthcare system.
Methods
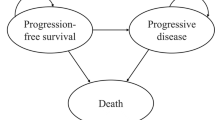
A Markov model was established to evaluate the cost-effectiveness of first-line treatment with TTP for patients with advanced or metastatic ESCC. Survival data were derived from the JUPITER-06 trial. The costs and utilities were gathered from the literature and a local database. The primary outcomes were total costs, quality-adjusted life year (QALY), and incremental cost-effectiveness ratios (ICERs) at a willingness-to-pay (WTP). One-way and probability sensitivity analyses were used to evaluate the robustness of the model.
Results
The total cost of TTP was $123,646.43 and gained 1.10 QALYs, while the paclitaxel and cisplatin (TP) chemotherapy group yielded 0.84 QALY at cost of $16,259.65. First-line TTP treatment yielded an incremental cost of $7,386.78 with an additional 0.26 QALY, providing an ICER of $28,348.42/QALY, which was lower than the WTP threshold ($36,257.91) in China.
Conclusions
TTP was likely more cost-effective than TP chemotherapy from the perspective of the Chinese healthcare system. This study may provide evidence required to establish decision-making criteria to support guidance for cost-effective selection of an immunotherapeutic regimen.



Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.10.3322/caac.21660.
Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous cell carcinoma. Gastroenterology. 2018;154:360–73. https://doi.org/10.1053/j.gastro.2017.08.023.10.1053/j.gastro.2017.08.023.
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41:1037–48. https://doi.org/10.1002/cac2.12197.10.1002/cac2.12197.
Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783–91. https://doi.org/10.1097/CM9.0000000000001474.10.1097/CM9.0000000000001474.
Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus. 2019;16:25–43. https://doi.org/10.1007/s10388-018-0642-8.10.1007/s10388-018-0642-8.
Petrasch S, Welt A, Reinacher A, Graeven U, König M, Schmiegel W. Chemotherapy with cisplatin and paclitaxel in patients with locally advanced, recurrent or metastatic oesophageal cancer. Br J Cancer. 1998;78:511–4. https://doi.org/10.1038/bjc.1998.524.10.1038/bjc.1998.524.
Zhang X, Shen L, Li J, Li Y, Li J, Jin M. A phase II trial of paclitaxel and cisplatin in patients with advanced squamous-cell carcinoma of the esophagus. Am J Clin Oncol. 2008;31:29–33. https://doi.org/10.1097/COC.0b013e3181131ca9.10.1097/COC.0b013e3181131ca9.
Constantinidou A, Alifieris C, Trafalis DT. Targeting programmed cell death -1 (PD-1) and Ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol Ther. 2019;194:84–106. https://doi.org/10.1016/j.pharmthera.2018.09.008.10.1016/j.pharmthera.2018.09.008.
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386:449–62. https://doi.org/10.1056/NEJMoa2111380.10.1056/NEJMoa2111380.
Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38:4138–48. https://doi.org/10.1200/jco.20.01888.10.1200/jco.20.01888.
Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (London, England). 2021;398:759–71. https://doi.org/10.1016/s0140-6736(21)01234-4.10.1016/s0140-6736(21)01234-4
Chau I, Doki Y, Ajani JA, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. JCO. 2021;39:LBA4001-LBA. https://doi.org/10.1200/JCO.2021.39.15_suppl.LBA4001.10.1200/JCO.2021.39.15_suppl.LBA4001
Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of Camrelizumab vs. placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326:916–25. https://doi.org/10.1001/jama.2021.12836.10.1001/jama.2021.12836.
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40:277-88.e3. https://doi.org/10.1016/j.ccell.2022.02.007.10.1016/j.ccell.2022.02.007.
Yamamoto S, Kato K. JUPITER-06 establishes immune checkpoint inhibitors as essential first-line drugs for the treatment of advanced esophageal squamous cell carcinoma. Cancer Cell. 2022;40:238–40. https://doi.org/10.1016/j.ccell.2022.02.009.10.1016/j.ccell.2022.02.009.
CSCO. The Chinese Society of Clinical Oncology(CSCO):Clinical guidelines for the diagnosis and treatment of esophageal squamous cell carcinoma, 2022. 2022.
Dolgin E. Bringing down the cost of cancer treatment. Nature. 2018;555:S26–9. https://doi.org/10.1038/d41586-018-02483-3.10.1038/d41586-018-02483-3.
Zhang Q, Wu P, He X, Ding Y, Shu Y. Cost-effectiveness analysis of camrelizumab vs. placebo added to chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China. Front Oncol. 2021;11:790373. https://doi.org/10.3389/fonQ3c.2021.790373.10.3389/fonc.2021.790373.
Zhu Y, Liu K, Ding D, Zhou Y, Peng L. Pembrolizumab plus chemotherapy as first-line treatment for advanced esophageal cancer: a cost-effectiveness analysis. Adv Ther. 2022;39:2614–29. 10.1007/s12325-022-02101-9.10.1007/s12325-022-02101-9.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103. https://doi.org/10.1001/jama.2016.12195.10.1001/jama.2016.12195.
BANK OF CHINA 2021. Foreign exchange rate. https://www.boc.cn/sourcedb/whpj/.
Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28. https://doi.org/10.1111/j.1524-4733.2004.75003.x.10.1111/j.1524-4733.2004.75003.x.
Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9:235–51. https://doi.org/10.1002/(sici)1099-1050(200004)9:3%3c235::aid-hec502%3e3.0.co;2-o.10.1002/(sici)1099-1050(200004)9:3%3c235::aid-hec502%3e3.0.co;2-o.
China. NBoso. Statistical Bulletin of National Economic and Social Development of the People’s Republic of China in 2021. Available online at http://wwwstatsgovcn/xxgk/sjfb/zxfb2020/202202/t20220228_1827971html (accessed Jul 01, 2022).
Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. https://doi.org/10.1186/1471-2288-11-139.10.1186/1471-2288-11-139.
Kohn CG, Zeichner SB, Chen Q, Montero AJ, Goldstein DA, Flowers CR. Cost-effectiveness of immune checkpoint inhibition in BRAF wild-type advanced melanoma. J Clin Oncol. 2017;35:1194–202. https://doi.org/10.1200/jco.2016.69.6336.10.1200/jco.2016.69.6336.
Chen X, Liang W, Wan N, Zhang L, Yang Y, Jiang J, et al. Cost-effectiveness analysis of gemcitabine plus cisplatin versus fluorouracil plus cisplatin for first-line treatment of recurrent or metastatic nasopharyngeal carcinoma. Oral Oncol. 2019;94:80–5. https://doi.org/10.1016/j.oraloncology.2019.04.022.10.1016/j.oraloncology.2019.04.022.
Diaby V, Adunlin G, Montero AJ. Survival modeling for the estimation of transition probabilities in model-based economic evaluations in the absence of individual patient data: a tutorial. Pharmacoeconomics. 2014;32:101–8. https://doi.org/10.1007/s40273-013-0123-9.10.1007/s40273-013-0123-9.
PLATFORM GPRTS. Guangdong public resources trading service platform: pharmaceutical procurement in Guangzhou. https://gpogzggzycn/. 2022.
Lin YT, Chen Y, Liu TX, Kuang F, Huang P. Cost-effectiveness analysis of camrelizumab immunotherapy versus docetaxel or irinotecan chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma. Cancer Manag Res. 2021;13:8219–30. https://doi.org/10.2147/cmar.S335515.10.2147/cmar.S335515.
Yang F, Fu Y, Kumar A, Chen M, Si L, Rojanasarot S. Cost-effectiveness analysis of camrelizumab in the second-line treatment for advanced or metastatic esophageal squamous cell carcinoma in China. Annals of translational medicine. 2021;9:1226. https://doi.org/10.21037/atm-21-1803.10.21037/atm-21-1803
Wu B, Ye M, Chen H, Shen JF. Costs of trastuzumab in combination with chemotherapy for HER2-positive advanced gastric or gastroesophageal junction cancer: an economic evaluation in the Chinese context. Clin Ther. 2012;34:468–79. https://doi.org/10.1016/j.clinthera.2012.01.012.10.1016/j.clinthera.2012.01.012.
Edlin R, Mccabe C, Hulme C, Hall P, Wright J. Cost effectiveness modelling for health technology assessment. J Springer Int Publishing. 2015. https://doi.org/10.1007/978-3-319-15744-3:pp.41-57.
Galluzzi L, Humeau J, Buque A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–41. https://doi.org/10.1038/s41571-020-0413-z.10.1038/s41571-020-0413-z.
Jin Z, Shen J, Wang C, Chen D, Zhang B, Zhang J, et al. Narrative review of pembrolizumab for the treatment of esophageal cancer: evidence and outlook. Annals of translational medicine. 2021;9:1189. https://doi.org/10.21037/atm-21-2804.10.21037/atm-21-2804
Liu CY, Lin CS, Shih CS, Huang YA, Liu CC, Cheng CT. Cost-effectiveness of minimally invasive esophagectomy for esophageal squamous cell carcinoma. World J Surg. 2018;42:2522–9. https://doi.org/10.1007/s00268-018-4501-5.10.1007/s00268-018-4501-5.
Wu B, Wang Z, Zhang Q. Age at initiation and frequency of screening to prevent esophageal squamous cell carcinoma in high-risk regions: an economic evaluation. Cancer Prev Res (Phila). 2020;13:543–50. https://doi.org/10.1158/1940-6207.Capr-19-0477.10.1158/1940-6207.Capr-19-0477.
Zhan M, Zheng H, Yang Y, Xu T, Li Q. Cost-effectiveness analysis of neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced esophageal squamous cell carcinoma based on the NEOCRTEC5010 trial. Radiotherapy Oncol. 2019;141:27–32. https://doi.org/10.1016/j.radonc.2019.07.031.10.1016/j.radonc.2019.07.031.
Zheng Z, Lin J, Zhu H, Cai H. Cost-effectiveness analysis of pembrolizumab plus chemotherapy vs. chemotherapy alone as first-line treatment in patients with esophageal squamous cell carcinoma and PD-L1 CPS of 10 or more. Front Public Health. 2022;10:893387. https://doi.org/10.3389/fpubh.2022.893387.10.3389/fpubh.2022.893387.
Zhang PF, Xie D, Li Q. Cost-effectiveness analysis of nivolumab in the second-line treatment for advanced esophageal squamous cell carcinoma. Future Oncol (Lond Engl). 2020;16:1189–98. https://doi.org/10.2217/fon-2019-0821.10.2217/fon-2019-0821.
Zhan M, Xu T, Zheng H, He Z. Cost-effectiveness analysis of pembrolizumab in patients with advanced esophageal cancer based on the KEYNOTE-181 study. Front Public Health. 2022;10: 790225. https://doi.org/10.3389/fpubh.2022.790225.10.3389/fpubh.2022.790225.
Zhu C, Xing XX, Wu B, Liang G, Han G, Lin CX, et al. Cost-effectiveness analysis of camrelizumab plus chemotherapy vs. chemotherapy alone as the first-line treatment in patients with IIIB-IV non-squamous non-small cell lung cancer (NSCLC) without EGFR and ALK alteration from a perspective of Health - Care System in China. Front Pharmacol. 2021;12:735536. https://doi.org/10.3389/fphar.2021.735536.10.3389/fphar.2021.735536.
Acknowledgements
Funding
This work and publication was supported by the Hospital Pharmaceutical Research Foundation of Guangdong Province (No.2022A35) and Pharmaceutical Scientific Research Foundation of Guangdong Provincial Hospital Association (No.2021YXQN10).
Author Contributions
Rui Fang: Study concepts and design, manuscript preparation, manuscript editing, literature research, experimental studies/data analysis. Suiqiong Wang: Manuscript editing, literature research. Yongqian Liu: Manuscript editing. Jun Xu: Guarantor of integrity of the entire study, manuscript editing.
Disclosures
Rui Fang, Suiqiong Wang, Yongqian Liu, and Jun Xu declare that they have no competing interests.
Compliance with Ethics Guidelines
This economic analysis was based on a literature review and modeling techniques, and this study did not require approval from an Institutional Research Ethics Board.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, R., Wang, S., Liu, Y. et al. Cost-Effectiveness Analysis of Toripalimab Plus Paclitaxel and Cisplatin as First-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma. Adv Ther 40, 1019–1030 (2023). https://doi.org/10.1007/s12325-022-02402-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02402-z