Abstract
Background
Schizophrenia is a chronic mental disorder associated with substantial morbidity and mortality affecting 0.25–1.6% of adults in the USA. Antipsychotic treatment is the standard of care for schizophrenia, but real-world treatment patterns and associated costs have not been systematically reviewed.
Objective
We conducted a systematic review to summarize treatment patterns and associated costs related to oral antipsychotic treatment of patients with schizophrenia in the USA.
Data Sources
We searched Medline (via PubMed) and Embase to identify relevant observational studies published from January 1, 2008, to June 1, 2018; costs were converted to 2018 US dollars.
Study Eligibility
Observational, real-world studies reporting on patterns of treatment and/or associated costs for adult patients with schizophrenia treated with oral antipsychotics in the USA were included.
Results
Eighty-one studies were identified. Frequently prescribed oral second-generation antipsychotics were olanzapine (up to 50.9%), risperidone (up to 40.0%), and quetiapine (up to 30.7%). Suboptimal adherence was common across studies. Antipsychotic switching occurred in about half of patients, while antipsychotic combination therapy occurred in nearly 30%; all were associated with increased medication-related costs. Mean annual direct medical costs differed by treatment, with reported costs of $17,115 to $26,138 for patients treated with olanzapine, $18,395 for risperidone, and $17,656 to $28,101 for quetiapine.
Limitations
This systematic review is limited by the variations in definitions of schizophrenia-related clinical terms used between studies and by the inclusion of studies focused on only the US health care system.
Conclusions
In the treatment of schizophrenia, suboptimal adherence, antipsychotic switching, and antipsychotic augmentation were all associated with high costs of care in comparison to patients who were adherent and did not require antipsychotic switching or augmentation. These findings illustrate the need for the development of new treatments that address efficacy and adherence challenges of currently available therapies.
Plain Language Summary
Schizophrenia is a debilitating mental disorder that affects up to 1.6% of adults in the USA. Antipsychotic medications reduce symptoms of the disease, but many patients with schizophrenia are not fully adherent or choose to discontinue treatment entirely, increasing their risk of hospitalization. In others, efforts to achieve better symptom control or to avoid intolerable side effects may result in switching antipsychotic medications or adding additional medications, leading to higher medical treatment costs. The magnitude of these cost increases is unclear. This study sought to assess medical costs associated with antipsychotic treatment adherence, switching, and adding additional antipsychotics. We reviewed 81 studies published from January 2008 through June 2018 examining treatment adherence in patients with schizophrenia. We calculated rates of adherence, switching, and adding antipsychotics, as well as associated medical costs. Overall adherence to antipsychotic treatment was less than 50%, with up to 50% of patients switching medications and up to 29% adding an additional antipsychotic medication to their current treatment. Patients who were not treatment adherent incurred annual medical costs of $10,316 compared with $5723 in patients who were adherent. The costs of immediate or delayed switching of antipsychotic medications ranged from $21,922 to $28,232, while costs of adding an additional antipsychotic ranged from $24,045 to $29,344. These data suggest that suboptimal medication adherence, along with high rates of patient discontinuation and medication switching, lead to higher treatment costs in the management of patients with schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The objective of this systematic review was to summarize oral antipsychotic treatment patterns (e.g., switching, discontinuing, or augmenting antipsychotic medications) and associated costs among patients living with schizophrenia in the USA from real-world evidence. |
Oral antipsychotic medication costs are a significant proportion of the economic burden of schizophrenia, contributing 28–44% of total direct medical costs annually. |
Suboptimal adherence to oral antipsychotic medications was common: adherent patients had three times higher annual medication costs, whereas patients with suboptimal adherence had 50% higher annual inpatient costs. |
Switching or combining oral antipsychotic medications was also common, with total direct costs as high as $28,232 for patients who switched treatments and $29,344 for those who augmented their treatment. |
There remains an unmet need for new, efficacious antipsychotic medications that may improve adherence, decrease health care resource utilization, and lessen the cost burden associated with schizophrenia. |
Introduction
Schizophrenia is a serious, chronic mental health disorder that impacts individuals and society as a whole. Among adults in the USA, the estimated prevalence of schizophrenia and related psychotic disorders ranges from 0.25% to 1.6% of the population [1,2,3,4]. Previous reviews have found that schizophrenia is associated with a substantial economic burden on the US health care system that is estimated to be as high as $174 billion annually [5]. Drivers of the excess costs of schizophrenia include direct medical costs such as pharmacotherapy and inpatient and outpatient care, as well as indirect costs associated with unemployment and caregiving [3, 5].
Antipsychotic medications are the first-line treatment for schizophrenia and are effective at reducing the symptoms of the disease [6]. As such, antipsychotic pharmacotherapy comprises a substantial proportion of direct medical costs within the US health care system [3, 5]. However, many patients with schizophrenia are not fully adherent to their medications or choose to discontinue treatment entirely, increasing their risk of relapse and hospitalization [7, 8]. In other patients, efforts to achieve better symptom control or avoid intolerable side effects may result in switching antipsychotic medications or adding additional medications [8,9,10], leading to higher medical costs [11]. The magnitude of costs associated with these antipsychotic treatment outcomes within the US health care system is unclear.
While individual studies have assessed treatment patterns with the use of antipsychotic medications among patients with schizophrenia in the USA, the economic implications associated with different treatment patterns have not been reported in the literature. Previous systematic reviews have focused on specific subpopulations of patients living with schizophrenia (e.g., privately insured patients [12]) and have not assessed treatment patterns and costs for broader populations.
Objective
The objective of this systematic review was to identify and summarize real-world evidence for oral first-generation and second-generation antipsychotic (FGA and SGA, respectively) treatment patterns and associated costs among patients living with schizophrenia in the USA.
Methods
Data Sources and Search Strategy
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13]. The PRISMA checklist is included in Supplementary Table S1. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors; thus, review by an institutional review board was not applicable for this study.
Literature searches were conducted in Medline (via PubMed) and Embase using a combination of medical subject heading and free-text terms to identify English-language articles published from January 1, 2008, to June 1, 2018 (for full search details, see Supplementary Tables S2–S5). Database searches were supplemented by a review of abstracts from relevant scientific conferences (2016–2018), including the International Society for Pharmacoeconomics and Outcomes Research, American Psychiatric Association Annual Meeting, US Psychiatric Congress, and the Academy of Managed Care Pharmacy. A manual check of references in the bibliographies of previously published systematic reviews (2015–2018) was performed to ensure comprehensive identification of relevant articles.
Study Eligibility and Data Extraction
Eligibility for inclusion was determined using prespecified populations, interventions, comparisons, outcomes, and study design (PICOS) inclusion/exclusion criteria [14] (Supplementary Table S6). Observational, real-world studies reporting on patterns of treatment and/or associated costs for adult patients with schizophrenia treated with oral antipsychotics in the USA were included. Treatment patterns of long-acting injectable (LAI) antipsychotics were not the subject of this review.
Abstracts and full-text publications were screened by one reviewer (KS or AC), with independent review and confirmation of 20% of excluded abstracts and 100% of excluded full-text articles by a second reviewer (AM or RH). Data points from included studies were extracted by a single reviewer (KS, AC, or RH), with accuracy and presence of each data point confirmed by a second reviewer (AM or RH). Discrepancies were resolved through discussion with a third reviewer (AM or RH). Data were summarized for oral antipsychotic treatment patterns, including real-world use, adherence, medication discontinuation, switching, and augmentation (e.g., combination treatment with more than one antipsychotic) using qualitative, thematic, and narrative synthesis. Costs associated with treatment patterns were summarized where data were available. Because this systematic review was limited to observational, real-world studies, a risk-of-bias assessment was not conducted, as that assessment is focused mainly on randomized controlled trials that would be included in a meta-analysis.
Schizophrenia Terminology and Treatment Pattern Definitions
Results reported for treatment patterns and associated costs followed the definitions and terminologies that were used by study authors. Definitions of schizophrenia-related clinical terms, such as discontinuation, augmentation, switch, and treatment resistance, often varied among the studies. Table 1 summarizes the definitions of various treatment pattern-related terms included in this systematic review. Adherence was defined using one of two pharmacy-based proxy approaches: proportion of days covered (PDC) or medication possession ratio (MPR) [15]. Consistent with expert consensus, rates of 80% or higher were considered adherent [8].
Additionally, results from the initial Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study [16], published in late 2005, suggested that, while olanzapine was the most effective antipsychotic on the basis of time to all-cause discontinuation, it was also associated with the greatest amount of weight gain and the emergence of metabolic disturbances over time. Therefore, prescribing trends for oral SGAs were compared between observational studies that reported data derived before 2006 and those that reported data derived after 2006.
Cost Definitions and Costing Approach
Cost definitions were presented as reported by authors of included publications. Costs included in this review are direct medical costs. Cost data were converted to 2018 US dollars (USD) using an inflation factor calculated by the US Department of Labor Statistics Consumer Price Index. The inflation factor corresponding to average medical care in US cities for all urban consumers was applied [17] and costs were determined on the basis of the year(s) reported in source articles. If cost year was not reported, the publication year was used as a proxy.
Results
Study Identification
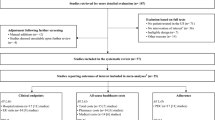
A total of 6605 unique records were identified. Of these, 603 abstracts were considered potentially eligible for inclusion, and full-text articles of their associated publications were reviewed. A total of 71 studies met inclusion criteria; 10 additional studies were identified from conference proceedings, resulting in a total of 81 included studies (Fig. 1).
Study Characteristics
Of the 81 included studies, most were retrospective in design (90%), and the remaining were prospective cohort (5%), cross-sectional (4%), or case control (1%) studies. A table of study characteristics is included in Supplementary Table S7. Medication adherence and information on treatment changes were the most commonly reported outcomes; both were reported in 41% of studies. Direct costs of drugs, treatment patterns, or outcomes of treatment were reported in 42% of studies, with some studies presenting costs stratified by medication type (50%), treatment change versus no treatment change (15%), treatment adherence versus suboptimal adherence (6%), and polypharmacy versus monotherapy (6%).
Direct Costs Associated with Oral Antipsychotic Treatment
Patterns of Use
Among the included studies, the most frequently reported oral SGAs were olanzapine, quetiapine, risperidone, and aripiprazole (Fig. 2). In these studies, 9.4–50.9% of patients in the study population took olanzapine [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], 11.5–30.7% took quetiapine [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], 2.0–40.0% took risperidone [18,19,20,21, 23, 24, 26,27,28,29,30,31,32,33,34], 4.0–21.5% took aripiprazole [18,19,20,21, 23, 24, 26,27,28,29,30,31,32, 35], and 0–7% took clozapine [20, 24,25,26, 31, 36]. In real-world studies published prior to 2006, olanzapine was the administered treatment for an estimated 16.0–50.9% [18, 21, 25, 27, 30, 32, 33] of patients making up the study population; however, this range dropped to below 20% after 2006 (9.4–17.4%) [20, 23, 24, 26, 28, 29, 31]. Conversely, prescribing trends for quetiapine and risperidone have remained relatively constant over time. Aripiprazole was approved by the US Food and Drug Administration in 2002, a time after data collection began for five studies included in this review. Excluding those studies, the reported proportion of patients treated with aripiprazole between 2008 and 2018 ranged from 8.7% to 21.5% [19, 20, 23, 24, 26, 28, 29, 31, 35].
Proportion of patients with schizophrenia prescribed oral SGAs in real-world evidence observational studies [18, 20, 21, 23,24,25,26,27,28,29,30,31,32,33, 63]. All studies reflected in this figure report on use of oral SGAs after 2006. The years in which generic drug versions became available are as follows: ARI, 2015; OLZ, 2011; QUE, 2016; RIS, 2008. ARI aripiprazole, OLZ olanzapine, QUE quetiapine, RIS risperidone, SGA second-generation antipsychotic
Direct Costs of Oral Antipsychotic Medications
Mean total annual direct costs by type of oral antipsychotic are summarized in Fig. 3 [28, 31, 32, 37,38,39,40,41,42]. Total annual direct costs ranged from $8465 for patients taking lurasidone to $28,101 for those taking quetiapine. In the subset of studies that did not provide cost estimates by a specific oral antipsychotic agent, total annual direct cost estimates ranged from $13,892 to $95,429.
Total annual direct costs by oral antipsychotic type in 2018 USD [28, 31, 32, 37,38,39,40,41,42]. ARI aripiprazole, CLZ clozapine, LUR lurasidone, OAT oral antipsychotic treatment (drugs not specified), OLZ olanzapine, PAL paliperidone, QUE quetiapine, RIS risperidone, ROA route of administration, USD US dollars
Annual medication costs are presented in Fig. 4 [28, 32, 33, 37, 38, 40, 41]. The largest estimated range came from studies that pooled results from all treatments ($936 to $9290). Those treated with olanzapine or quetiapine had similar ranges of annual medication costs. Estimates of annual medication cost of lurasidone, paliperidone, and aripiprazole originated from only one study [28].
Direct cost components associated with schizophrenia by agent are provided in Fig. 5 [28, 32, 38, 41, 43]. The proportions of medication costs contributing to annual total direct costs ranged from 30% for quetiapine to 70% for lurasidone. The proportion of inpatient costs attributable to individual antipsychotics was similar among medications, ranging from 37% to 47%, except for lurasidone. A similar finding was noted for outpatient costs, with ranges between 21% and 24%, except for lurasidone. Although associated with lower inpatient and outpatient costs relative to paliperidone, aripiprazole, risperidone, quetiapine, and olanzapine, medication costs for lurasidone were the highest observed, making up 70% of the total direct costs associated with it.
Direct Costs Associated with Oral Antipsychotic Treatment Adherence
Adherence and Discontinuation Patterns
Thirty-three studies assessed patient adherence to oral antipsychotic medications. When a PDC of 80% or higher was used, adherence was reported to be below 50% (range 9.0–33.2%) in 11 of 12 studies with 6–12 months of follow-up [34, 42, 44,45,46,47,48,49,50,51,52] (Table 2). These findings were consistent with adherence rates assessed by MPRs of 80% or higher (Table 2), with oral antipsychotic adherence of less than 50% in 5 of 7 studies over 6–12 months of follow-up [29, 45, 53, 54], and by MPRs of 70% or higher [20], with adherence ranges of 22.0–45.1%. Patient adherence rates for individual oral antipsychotics were not extensively characterized in the literature reviewed.
Twenty-one studies reported outcomes related to treatment discontinuation (Table 3) [18, 21, 24, 25, 29, 30, 38, 42, 45,46,47, 49, 50, 54,55,56,57,58,59,60,61]. The overall frequency of treatment discontinuation varied widely, due in part to different definitions used for discontinuation and different durations of follow-up (ranging from 6 months to 15 years). Among the 15 studies assessing discontinuation rates, 12 reported a discontinuation rate of greater than 50% over follow-up periods of 6 months to 15 years; overall discontinuation rates for oral antipsychotics were greater than 70% in four studies with follow-up periods of 6 months to 4.5 years [29, 47, 57, 59]. Among the oral SGAs, discontinuation rates were generally comparable.
Cost of Suboptimal Adherence
The distribution of major cost components in the treatment of schizophrenia differed according to adherence status (Table 4). Overall, cost differences for adherent patients relative to those with suboptimal adherence were driven by medication and inpatient costs. Patients with suboptimal adherence had higher annual all-cause inpatient costs ($10,316 vs $5723) and schizophrenia-related inpatient costs ($2812 vs $944) relative to those who were considered adherent [20]. This trend was confirmed by data on quarterly inpatient costs, which ranged from $2378 to $4347 in adherent patients compared with $3444 to $5342 in patients with suboptimal adherence [62]. Adherent patients had nearly three times the annual antipsychotic medication costs of those with suboptimal adherence ($1806 vs $559 in a Medicaid population study [62]; $3550 vs $1236 in a Medicare population study [20]), whereas patients with suboptimal adherence had annual inpatient costs, on average, that were approximately 50% higher than those of patients who were adherent.
Direct Costs Associated with Oral Antipsychotic Treatment Changes
Patterns of Treatment Restarting or Switching
Treatment changes for which data were available included restarting or switching treatment and antipsychotic combination therapy (Table 5). Twelve studies provided data on antipsychotic treatment restarts or switches [22, 24, 25, 27, 30, 37, 38, 53, 56, 59, 63, 64]. Of these studies, two reported the proportion of patients who restarted treatment, defined as a medication gap of at least 15 days followed by continuing the previously taken oral antipsychotic medication [22, 63]. Restarting rates were highest for olanzapine (61.5%) and risperidone (57.6%) and lower for quetiapine (38.3%) and FGAs (43.8%). Although treatment restarts were defined as a gap of at least 15 days, the mean times to restart for individual oral antipsychotic medications were 73 days for olanzapine, 72 for risperidone, 56 for quetiapine, and 99 for FGAs [63].
Treatment switches (defined variably across studies in terms of length of gap between prescriptions) occurred in 2.7% to 50% of patients over time periods ranging from 6 to 24 months [22, 24, 25, 27, 38, 53, 56, 59, 63,64,65,66]. The average time to switch across all oral antipsychotics ranged from 47 to 282.9 days [24, 56, 63]. Treatment switching rates over follow-up periods from 6 months to 2 years ranged from 11.0% to 20.0% with olanzapine, 12.8% to 30.0% with risperidone, 10.6% to 19.4% with quetiapine, and 8.8% with aripiprazole [22, 24, 59, 63, 66]. Time to medication switch was shorter for patients receiving FGAs (47 days) compared with those receiving olanzapine, risperidone, or quetiapine (64–68 days) [63]. Among patients treated with olanzapine or risperidone, those on risperidone were more likely to switch medications within 2 years (30.0% vs 20.0%) [59]. For olanzapine, risperidone, and quetiapine, when switches occurred, they more commonly occurred after a break in treatment versus an acute switch within days of discontinuation, regardless of the index SGA taken [63].
Cost of Treatment Restarting or Switching
In patients with an antipsychotic treatment gap of at least 15 days who then restarted their previous oral antipsychotic medication, mean annual total costs ranged from $17,278 in patients taking olanzapine to $22,199 in patients taking quetiapine [22, 67] (Table 6). For those switching from one oral medication to another, defined as a change while still on active treatment or within 15 days of terminating the previous treatment and with discontinuation of the previous antipsychotic medication within 60 days, mean total costs ranged from $23,346 to $28,232 [22, 67]. Patients with a delayed switch (i.e., no antipsychotic medication for at least 15 days followed by initiation of a different antipsychotic medication) had mean total direct costs similar to those of patients who switched without a gap in treatment, ranging from $21,922 to $24,265 [22, 67].
Patterns of Combination Treatment
Rates of combination treatment with an additional oral antipsychotic (Table 5) ranged from 9.4% to 29.2% over 6 to 12 months of follow-up [51, 63, 66, 68]. Data regarding specific combinations of antipsychotic medications taken by patients were limited. One study reported that, among 968 patients receiving antipsychotic combination treatment, quetiapine and risperidone were the most frequently used combination (9.9%), followed by quetiapine and aripiprazole (9.7%) [24]. Compared with patients treated with quetiapine, those treated with olanzapine had lower rates of combination treatment with any additional antipsychotic or specifically with an additional SGA [66]. Rates of combination treatment with non-antipsychotic psychotropic medications were substantially higher than rates of combination treatment with an additional oral antipsychotic, ranging from 52.0% to 67.7% over 12 months of follow-up [34, 51].
Cost of Combination Treatment
Total annual costs associated with augmentation of treatment with an additional antipsychotic medication ranged from a mean of $24,045 in those treated with olanzapine to $29,344 in those treated with risperidone (Table 6). The difference in costs associated with augmentation is likely attributable to increased medication costs [22, 67]. Clozapine monotherapy was associated with an average estimated total cost reduction of $23,025 per year versus antipsychotic combination treatment. These cost reductions were attributed to lower use of mental disorder-related and schizophrenia-related emergency department services. There were no differences for clozapine monotherapy versus combination antipsychotic treatment in terms of the likelihood of hospitalization or all-cause emergency department visits [69]. Additionally, cost related to adding the nonindexed antipsychotic medication(s) was lower for olanzapine than for quetiapine (33.7% and up to 64.6% of the total annual medication costs, respectively). The main cost driver was the type of co-prescription added (FGA vs SGA) [33].
Costs of Any Antipsychotic Treatment Change
Overall, medication costs were a driver of annual total direct costs in patients experiencing a treatment change, accounting for 28% to 44% of this overall expenditure [22, 67, 70], with the higher end of the range represented by patients who augmented their treatment owing to inadequate symptom control (Table 7) [22, 67]. Mean inpatient costs associated with a treatment change were generally a small portion (5–14%) of total annual costs associated with caring for patients with schizophrenia, the majority of which were psychiatric hospital care costs. Acute hospital costs ranged from a mean of $354 to $949 annually, while psychiatric hospital costs averaged between $735 and $2551 annually. However, it should be noted that this figure excluded one study as an outlier that reported on patients switching from quetiapine [22]; when this study was included, the range of psychiatric hospital costs was $735 to $23,835 per annum. Ambulatory care costs accounted for approximately 7% to 11% of total annual costs.
Discussion
This systematic review identified and synthesized available evidence associated with real-world treatment patterns in schizophrenia to provide critical information regarding the extent of disease burden and economic implications associated with these treatment patterns. Annual medication-related treatment costs were highly variable, even when stratified by drug, because of differences in study populations, severity of disease (e.g., relapsed vs stable), setting (e.g., outpatient vs hospitalized patients), or phase of treatment (e.g., switching vs restarting). Despite the wide range across studies, medication costs for oral antipsychotics contributed 28% to 44% of annual mean total direct costs and represent a significant proportion of the economic burden of schizophrenia [22, 67, 70].
Although continuous long-term pharmacologic treatment is a goal in the management of schizophrenia, real-world studies indicated that adherence to oral antipsychotics was commonly below 50% and treatment discontinuations were high (greater than 50%) [29, 34, 42, 44,45,46,47,48,49,50,51,52,53,54].
Suboptimal medication adherence has been linked to relapse and rehospitalization [71,72,73], with one study reporting that 68% of rehospitalization costs were attributed to loss of efficacy and 32% were attributed to lack of adherence [71]. In this review, patients with suboptimal adherence to their oral antipsychotic medication had higher medical costs that were driven by increased inpatient care costs [20, 62].
Furthermore, up to half of patients switched from their index antipsychotic medication to a different agent, while approximately 30% augmented with an additional antipsychotic (i.e., combination antipsychotic therapy), highlighting the need for residual symptom control and/or the treatment of other psychiatric comorbidities in this population. Although these strategies may improve treatment response, both options were associated with higher costs of care. For patients who switched oral antipsychotic medications, total direct costs were as high as $24,265 per patient, while the cost of augmenting was as high as $29,344. Regardless of the pattern of switch that occurs, switching is costly. These issues highlight the importance of first-line antipsychotic treatments that are both highly effective and well tolerated to avoid unnecessary medication discontinuations, switches, or treatment augmentation and their associated increased treatment costs.
Limitations
As is the case with all systematic reviews, this review is limited by potential reporting and publication biases, restricting the scope of patient characteristics, outcome definitions, and time periods reported. In addition, definitions of schizophrenia-related clinical terms often varied between studies. Some outcomes (e.g., switching or discontinuation rates) were not standardized into person-months of exposure but are presented as aggregates with variable follow-up times. While discontinuation from medication was commonly reported, the reason for stopping medication or the subsequent next step in patients’ treatment journeys were rarely available, reflecting that many included studies utilized administrative claims databases to elucidate costs, and reasons for discontinuation are generally not coded as part of the claim. Additionally, there is not enough information on differences between settings of care to allow for comparisons between these outcomes.
Because only a subset of studies contributed to cost estimates of antipsychotic treatment patterns, the range of estimates should be interpreted with caution. As reported in this review, treatment costs are highly variable across patients. Patients with schizophrenia who have suboptimal adherence or who discontinue their medication have higher inpatient costs due to episodes of relapse and hospitalization [7]. Furthermore, given the uncontrolled nature of the studies examined, confounding by indication cannot be ruled out.
Another limitation is that only direct costs related to oral antipsychotic medications were considered. Disease-related indirect costs comprise most of the excess economic burden of schizophrenia [3, 5], and the loss of productivity by patients and caregivers and premature patient mortality are substantial contributors to the societal costs of schizophrenia [3, 74]. Also, this review did not include LAI antipsychotics in estimates of medication costs, although some studies have found that these agents may be associated with lower inpatient but higher pharmacy costs [75]. As the review was restricted to the US health care system, the economic burden of schizophrenia in other countries was not evaluated.
Furthermore, none of the articles included in this review reported on prescribing patterns or cost differences after a branded product became available as a generic product. This review builds on previous research by focusing on data specific to treatment patterns for schizophrenia in the USA, but without a limitation to population, as was the case with a recent systematic review that restricted inclusion to studies in privately insured patients [12]. By including additional evidence published after previous reviews, we aimed to provide a broader understanding of treatment patterns and costs associated with the treatment of schizophrenia.
Conclusions
This systematic review of oral antipsychotic treatment patterns and associated costs among patients with schizophrenia in the USA found that antipsychotic medication adherence is low and discontinuation rates are high in this population. Therefore, many patients living with schizophrenia have symptoms that may not be optimally managed, leading to higher treatment costs when subsequent efforts aimed at reducing those symptoms include switches in treatment and/or augmentation with additional agents. Suboptimal adherence in these patients contributes to greater economic burden relative to those who are adherent to medications. Despite the number of available oral antipsychotics for schizophrenia, there remains an unmet need for new, highly efficacious treatments that may improve adherence. Such treatments may decrease health care resource utilization and the overall cost burden associated with schizophrenia over time.
References
Regier DA, Narrow WE, Rae DS, et al. The de facto US mental and addictive disorders service system. Epidemiologic catchment area prospective 1-year prevalence rates of disorders and services. Arch Gen Psychiatry. 1993;50:85–94.
Kessler RC, Birnbaum H, Demler O, et al. The prevalence and correlates of nonaffective psychosis in the National Comorbidity Survey Replication (NCS-R). Biol Psychiatry. 2005;58:668–76.
Desai PR, Lawson KA, Barner JC, Rascati KL. Estimating the direct and indirect costs for community-dwelling patients with schizophrenia. J Pharm Health Serv Res. 2013;4:187–94.
Wu EQ, Shi L, Birnbaum H, Hudson T, Kessler R. Annual prevalence of diagnosed schizophrenia in the USA: a claims data analysis approach. Psychol Med. 2006;36:1535–40.
Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77:764–71.
Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394:939–51.
Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10:2.
Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(Suppl 4):1–46; quiz 7–8.
American Psychiatric Association. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Washington, DC: American Psychiatric Association; 2021.
Correll CU, Martin A, Patel C, et al. Systematic literature review of schizophrenia clinical practice guidelines on acute and maintenance management with antipsychotics. NPJ Schizophr. 2022;8:5.
Faries DE, Ascher-Svanum H, Nyhuis AW, Kinon BJ. Clinical and economic ramifications of switching antipsychotics in the treatment of schizophrenia. BMC Psychiatry. 2009;9:54.
Zhang W, Amos TB, Gutkin SW, et al. A systematic literature review of the clinical and health economic burden of schizophrenia in privately insured patients in the United States. Clinicoecon Outcomes Res. 2018;10:309–20.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097
Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). Cochrane, 2019. www.training.cochrane.org/handbook. Accessed Apr 29, 2020.
Karve S, Cleves MA, Helm M, et al. Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia. Value Health. 2009;12:989–95.
Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23.
US Bureau of Labor Statistics. Consumer Price Index. US Department of Labor, 2020. https://www.bls.gov/cpi/. Accessed Apr 29, 2020.
Mullins CD, Obeidat NA, Cuffel BJ, Naradzay J, Loebel AD. Risk of discontinuation of atypical antipsychotic agents in the treatment of schizophrenia. Schizophr Res. 2008;98:8–15.
DiBonaventura MC, Panish J, Kenworthy D, Wagner JS, Dirani R. The association of well-being, productivity and resource use among community-dwelling patients with schizophreniausing atypical antipsychotics. J Pharm Health Serv Res. 2010;1:181–7.
Offord S, Lin J, Wong B, Mirski D, Baker RA. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenia patients with Medicare coverage. Commun Ment Health J. 2013;49:625–9.
Broder MS, Bates JA, Jing Y, et al. Association between second-generation antipsychotic medication half-life and hospitalization in the community treatment of adult schizophrenia. J Med Econ. 2012;15:105–11.
Chen L, McCombs JS, Park J. The impact of atypical antipsychotic medications on the use of health care by patients with schizophrenia. Value Health. 2008;11:34–43.
Lafeuille MH, Tandon N, Tiggelaar S, et al. Economic impact in Medicaid beneficiaries with schizophrenia and cardiometabolic comorbidities treated with once-monthly paliperidone palmitate vs. oral atypical antipsychotics. Drugs Real World Outcomes. 2018;5:81–90.
Fisher MD, Reilly K, Isenberg K, Villa KF. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC Psychiatry. 2014;14:341.
Farley JF. Medicaid prescription cost containment and schizophrenia: a retrospective examination. Med Care. 2010;48:440–7.
Berkowitz RL, Patel U, Ni Q, Parks JJ, Docherty JP. The impact of the clinical antipsychotic trials of intervention effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73:498–503.
Law MR, Soumerai SB, Ross-Degnan D, Adams AS. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008;69:47–53.
Jiang Y, Ni W. Health care utilization and treatment persistence associated with oral paliperidone and lurasidone in schizophrenia treatment. J Manag Care Spec Pharm. 2015;21:780–92.
Rajagopalan K, Wade S, Meyer N, Loebel A. Real-world adherence assessment of lurasidone and other oral atypical antipsychotics among patients with schizophrenia: an administrative claims analysis. Curr Med Res Opin. 2017;33:813–20.
Kreyenbuhl J, Slade EP, Medoff DR, et al. Time to discontinuation of first- and second-generation antipsychotic medications in the treatment of schizophrenia. Schizophr Res. 2011;131:127–32.
Manuel JI, Essock SM, Wu Y, Pangilinan M, Stroup S. Factors associated with initiation on clozapine and on other antipsychotics among Medicaid enrollees. Psychiatr Serv. 2012;63:1146–9.
Seabury SA, Goldman DP, Kalsekar I, et al. Formulary restrictions on atypical antipsychotics: impact on costs for patients with schizophrenia and bipolar disorder in Medicaid. Am J Manag Care. 2014;20:e52-60.
Zhu B, Ascher-Svanum H, Faries DE, Correll CU, Kane JM. Cost of antipsychotic polypharmacy in the treatment of schizophrenia. BMC Psychiatry. 2008;8:19.
El Khoury A, Joshi K, Brouillette M, Kalyanaraman A. Treatment patterns in schizophrenia patients initiated on paliperidone palmitate long-acting injectable in a medicaid population [abstract]. Presented at Annual Meeting of the American Psychological Association; August 9–12, 2018; San Francisco, CA.
Offord S, Lin J, Mirski D, Wong B. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther. 2013;30:286–97.
Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68:579–86.
Yu AP, Atanasov P, Ben-Hamadi R, et al. Resource utilization and costs of schizophrenia patients treated with olanzapine versus quetiapine in a Medicaid population. Value Health. 2009;12:708–15.
Pesa JA, Doshi D, Wang L, Yuce H, Baser O. Health care resource utilization and costs of California Medicaid patients with schizophrenia treated with paliperidone palmitate once monthly or atypical oral antipsychotic treatment. Curr Med Res Opin. 2017;33:723–31.
Pesa JA, Muser E, Montejano LB, Smith DM, Meyers OI. Costs and resource utilization among Medicaid patients with schizophrenia treated with paliperidone palmitate or oral atypical antipsychotics. Drugs Real World Outcomes. 2015;2:377–85.
Lafeuille MH, Grittner AM, Fortier J, et al. Comparison of rehospitalization rates and associated costs among patients with schizophrenia receiving paliperidone palmitate or oral antipsychotics. Am J Health Syst Pharm. 2015;72:378–89.
Baser O, Xie L, Pesa J, Durkin M. Healthcare utilization and costs of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J Med Econ. 2015;18:357–65.
Young-Xu Y, Duh MS, Muser E, et al. Impact of paliperidone palmitate versus oral atypical antipsychotics on health care resource use and costs in veterans with schizophrenia. J Clin Psychiatry. 2016;77:e1332–41.
Chitnis A, Sun SX, Dixit S, et al. Changes in patterns of utilization and cost of health care services associated with initiation of asenapine for the treatment of schizophrenia in adults. Manag Care. 2015;24:58–64.
Amos T, El Khoury A, Vlahiotis A, et al. Treatment patterns and healthcare resource utilization among young adults with schizophrenia treated with paliperidone palmitate or oral atypical antipsychotics [abstract PMH62]. Value Health. 2018;21:S190.
Anderson JP, Icten Z, Alas V, Benson C, Joshi K. Comparison and predictors of treatment adherence and remission among patients with schizophrenia treated with paliperidone palmitate or atypical oral antipsychotics in community behavioral health organizations. BMC Psychiatry. 2017;17:346.
Campagna EJ, Muser E, Parks J, Morrato EH. Methodological considerations in estimating adherence and persistence for a long-acting injectable medication. J Manag Care Spec Pharm. 2014;20:756–66.
Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21:127–34.
Joshi K, Lafeuille MH, Tandon N. Treatment patterns in schizophrenia patients initiated on paliperidone palmitate long-acting injectable in a medicaid population [abstract]. Presented at Annual Meeting of the American Psychological Association; August 9–12, 2018; San Francisco, CA.
MacEwan JP, Forma FM, Shafrin J, et al. Patterns of adherence to oral atypical antipsychotics among patients diagnosed with schizophrenia. J Manag Care Spec Pharm. 2016;22:1349–61.
Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21:754–68.
Pilon D, Muser E, Lefebvre P, et al. Adherence, healthcare resource utilization and Medicaid spending associated with once-monthly paliperidone palmitate versus oral atypical antipsychotic treatment among adults recently diagnosed with schizophrenia. BMC Psychiatry. 2017;17:207.
Pilon D, Tandon N, Lafeuille MH, et al. Treatment patterns, health care resource utilization, and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther. 2017;39:1972-85.e2.
Berger A, Edelsberg J, Sanders KN, et al. Medication adherence and utilization in patients with schizophrenia or bipolar disorder receiving aripiprazole, quetiapine, or ziprasidone at hospital discharge: a retrospective cohort study. BMC Psychiatry. 2012;12:99.
Panish J, Karve S, Candrilli SD, Dirani R. Association between adherence to and persistence with atypical antipsychotics and psychiatric relapse among US Medicaid-enrolled patients with schizophrenia. J Pharm Health Serv Res. 2013;4:29–39.
Davis MC, Fuller MA, Strauss ME, Konicki PE, Jaskiw GE. Discontinuation of clozapine: a 15-year naturalistic retrospective study of 320 patients. Acta Psychiatr Scand. 2014;130:30–9.
Xiao Y, Muser E, Lafeuille MH, et al. Impact of paliperidone palmitate versus oral atypical antipsychotics on healthcare outcomes in schizophrenia patients. J Comp Eff Res. 2015;4:579–92.
Horvitz-Lennon M, Donohue JM, Lave JR, Alegría M, Normand SL. The effect of race-ethnicity on the comparative effectiveness of clozapine among Medicaid beneficiaries. Psychiatr Serv. 2013;64:230–7.
Mohamed S, Rosenheck R, Harpaz-Rotem I, Leslie D, Sernyak MJ. Duration of pharmacotherapy with long-acting injectable risperidone in the treatment of schizophrenia. Psychiatr Q. 2009;80:241–9.
Kilzieh N, Todd-Stenberg JA, Kennedy A, Wood AE, Tapp AM. Time to discontinuation and self-discontinuation of olanzapine and risperidone in patients with schizophrenia in a naturalistic outpatient setting. J Clin Psychopharmacol. 2008;28:74–7.
Citrome L, Reist C, Palmer L, et al. Impact of real-world ziprasidone dosing on treatment discontinuation rates in patients with schizophrenia or bipolar disorder. Schizophr Res. 2009;115:115–20.
Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173:166–73.
Farley JF, Hansen RA, Yu-Isenberg KS, Maciejewski ML. Antipsychotic adherence and its correlation to health outcomes for chronic comorbid conditions. Prim Care Companion CNS Disord. 2012;14. https://doi.org/10.4088/PCC.11m01324.
Chen L, McCombs JS, Park J. Duration of antipsychotic drug therapy in real-world practice: a comparison with CATIE trial results. Value Health. 2008;11:487–96.
Wilk JE, West JC, Marcus SC, et al. Family contact and the management of medication non-adherence in schizophrenia. Commun Ment Health J. 2008;44:377–80.
Martin BC, Wiley-Exley EK, Richards S, et al. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. 2009;43:36–44.
Yu AP, Ben-Hamadi R, Birnbaum HG, et al. Comparing the treatment patterns of patients with schizophrenia treated with olanzapine and quetiapine in the Pennsylvania Medicaid population. Curr Med Res Opin. 2009;25:755–64.
Thomas KL, Jiang Y, McCombs JS. Clozapine revisited: Impact of clozapine vs olanzapine on health care use by schizophrenia patients on Medicaid. Ann Clin Psychiatry. 2015;27:90–9.
Joshi K, Kamstra R, Pilon D, Lefebvre P, Emond B. Treatment patterns and Medicaid spending in comorbid schizophrenia populations: once-monthly paliperidone palmitate versus oral atypical antipsychotics [abstract]. Presented at Annual Meeting of the American Psychiatric Association; May 20–24, 2017; San Diego, CA.
Velligan DI, Carroll C, Lage MJ, Fairman K. Outcomes of medicaid beneficiaries with schizophrenia receiving clozapine only or antipsychotic combinations. Psychiatr Serv. 2015;66:127–33.
Durkin M, Song X, Tran O, et al. Two-year acute care utilization and cost outcomes for schizophrenia patients in medicaid treated with paliperidone palmitate or oral atypical antipsychotic [abstract F14]. J Manag Care Spec Pharm. 2017;23(3-a suppl):S54.
Weiden PJ, Olfson M. Cost of relapse in schizophrenia. Schizophr Bull. 1995;21:419–29.
Haywood TW, Kravitz HM, Grossman LS, et al. Predicting the “revolving door” phenomenon among patients with schizophrenic, schizoaffective, and affective disorders. Am J Psychiatry. 1995;152:856–61.
Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161:692–9.
Olfson M, Gerhard T, Huang C, Crystal S, Stroup TS. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiat. 2015;72:1172–81.
Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77:1–24.
Gilmer TP, Ojeda VD, Barrio C, et al. Adherence to antipsychotics among Latinos and Asians with schizophrenia and limited English proficiency. Psychiatr Serv. 2009;60:175–82.
Wang CC, Maciejewski ML, Rao JK, et al. Examining the relationship between adjunctive psychotherapy use and antipsychotic persistence and hospitalization. Adm Policy Ment Health. 2014;41:598–607.
Lang K, Meyers JL, Korn JR, et al. Medication adherence and hospitalization among patients with schizophrenia treated with antipsychotics. Psychiatr Serv. 2010;61:1239–47.
Stephenson JJ, Tunceli O, Gu T, et al. Adherence to oral second-generation antipsychotic medications in patients with schizophrenia and bipolar disorder: physicians' perceptions of adherence vs. pharmacy claims. Int J Clin Pract. 2012;66:565–73.
Haynes VS, Zhu B, Stauffer VL, et al. Long-term healthcare costs and functional outcomes associated with lack of remission in schizophrenia: a post-hoc analysis of a prospective observational study. BMC Psychiatry. 2012;12:222.
Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157:259–63.
Ascher-Svanum H, Peng X, Faries D, Montgomery W, Haddad PM. Treatment patterns and clinical characteristics prior to initiating depot typical antipsychotics for nonadherent schizophrenia patients. BMC Psychiatry. 2009;9:46.
Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Geographic and clinical variation in clozapine use in the United States. Psychiatr Serv. 2014;65:186–92.
Correll C, Brevig T, Brain C. Burden and pharmacotherapy of treatment resistant schizophrenia: a survey among U.S. psychiatrists [poster]. Presented at Annual Meeting of the American Psychological Association; August 9–12, 2018; San Francisco, CA.
Cullen BA, McGinty EE, Zhang Y, et al. Guideline-concordant antipsychotic use and mortality in schizophrenia. Schizophr Bull. 2013;39:1159–68.
Greene M, Yan T, Chang E, Hartry A, Broder MS. Medication adherence and discontinuation in medicaid patients with schizophrenia initiating a long acting injectable antipsychotic versus those who change to a different oral antipsychotic [abstract PMH58]. Value Health. 2017;20:A302.
Acknowledgements
Funding
This manuscript (including the journal’s Rapid Service and Open Access fees) was funded by Alkermes, Inc. The sponsor participated in manuscript review and the decision to submit the paper for publication.
Medical Writing and Editorial Assistance
Medical writing and editorial support were provided by John H. Simmons, MD, Omar H. Cabrera, PhD, and Teri O’Neill of Peloton Advantage, LLC, an OPEN Health company, and funded by Alkermes, Inc.
Authorship
All authors met the International Committee of Medial Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors were involved in the design of the analysis, planning of data analysis, and interpretation of the data. All authors were involved in writing the manuscript and read and approved the manuscript prior to submission. All authors collaborated in the preparation of the manuscript and critically reviewed and provided revisions to the manuscript.
Disclosures
Michael J. Doane is an employee of Alkermes, Inc., and may own stock in the company. Leona Bessonova (currently affiliated with Intercept Pharmaceuticals), Amy K. O’Sullivan (currently affiliated with Ontada), and Peter J. Weiden (currently affiliated with Karuna Therapeutics) were employees of Alkermes, Inc., at the time the study was conducted and may own stock in the company. Amber Martin, Rachel Hughes, Kassandra Snook, and Allie Cichewicz are employees of Evidera, a research and consulting firm in the pharmaceutical industry. As salaried employees of Evidera, they work with a variety of companies and are precluded from accepting any payment or honoraria directly from those companies for services rendered. Evidera received payment from Alkermes, Inc., for collaboration on this project. Philip D. Harvey has received consulting fees or travel reimbursements from Acadia Pharma, Alkermes, Bio Excel, Boehringer Ingelheim, Intra-Cellular Therapies, Minerva Pharma, Regeneron Pharma, and Sunovion Pharma during the past year. He receives royalties from the Brief Assessment of Cognition in Schizophrenia. He is Chief Scientific Officer of i-Function, Inc. He has received research grants from Takeda and from the Stanley Medical Research Foundation.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors; thus, review by an institutional review board was not applicable for this study.
Data Availability
All data generated or analyzed during this study are included in this published article and as supplementary material files.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Martin, A., Bessonova, L., Hughes, R. et al. Systematic Review of Real-World Treatment Patterns of Oral Antipsychotics and Associated Economic Burden in Patients with Schizophrenia in the United States. Adv Ther 39, 3933–3956 (2022). https://doi.org/10.1007/s12325-022-02232-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02232-z