Abstract
Introduction
A systematic literature review (SLR) and network meta-analysis (NMA) were conducted to evaluate the comparative efficacy of brolucizumab relative to other anti-vascular endothelial growth factor (VEGF) treatments for neovascular age-related macular degeneration (nAMD) at 1 and 2 years, and overall safety and injection frequency of each treatment.
Methods
An SLR identifying randomized controlled trials (RCTs) published before June 2021 according to a pre-specified protocol was followed by a Bayesian NMA to compare brolucizumab (6 mg q12w/q8w) against sham and all relevant anti-VEGF regimens. Pooled mean injection frequency, serious adverse ocular events, and discontinuation rates were estimated for each treatment regimen.
Results
Nineteen RCTs were included in NMA base-case analysis. Brolucizumab (6 mg q12w/q8w) with loading-phase (LP) demonstrated superior best-corrected visual acuity (BCVA) gains to sham both at year 1 (mean difference 16.8 [95%CrI 13.3, 20.4]) and year 2 (mean difference 21.2 [95%CrI 17.4, 25.0]) and was comparable to other anti-VEGFs. Brolucizumab (6 mg q12w/q8w) also showed superior retinal thickness reduction to most comparators including ranibizumab (0.5 mg q4w; year 1 mean difference − 50.1 [95%CrI − 70.3, − 29.8]; year 2 mean difference − 49.5 [95%CrI − 70.8, − 28.6]), aflibercept (2 mg q8w; year 1 mean difference − 39.7 [95%CrI − 52.9, − 26.4]; year 2 mean difference − 35.0 [95%CrI − 49.1, − 21.4]), and faricimab (6 mg q16w/q8w; year 1 mean difference − 27.6 [95%CrI − 42.3, − 12.8]). Brolucizumab (6 mg q12w/q8w) showed similar rates of treatment discontinuation and serious and overall adverse events (both years). At year 2, pooled annualized injection frequency was lowest for brolucizumab (6 mg q12w/q8w) and highest for ranibizumab (0.5 mg q4w) at 5.7 and 11.5 injections annually, respectively.
Conclusion
Among all licensed anti-VEGF treatments, brolucizumab showed superior reduction in retinal thickness and comparable BCVA gains and discontinuation rates, despite having the lowest injection frequency. The current study provides the most up-to-date, robust comparison of treatments for nAMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Current pharmacologic therapy for neovascular age-related macular degeneration (nAMD) involves intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents. |
We conducted a network meta-analysis (NMA) comparing the efficacy and safety of brolucizumab to other anti-VEGF treatments in patients with nAMD. |
By combining direct and indirect evidence, NMA makes it possible to simultaneously compare multiple treatments that were not compared in head-to-head trials. |
What was learned from the study? |
Among all licensed anti-VEGF treatments, brolucizumab showed superior reduction in retinal thickness and comparable visual acuity gains and discontinuation rates, despite having the lowest injection frequency. |
The current NMA provides the most up-to-date, robust comparison of treatments for nAMD. |
Introduction
Age-related macular degeneration (AMD) is the main cause of severe visual loss and blindness in all high-income countries [1]. AMD progresses slowly over on average 10 years from early to intermediate to late stage disease which can include dry, or non-exudative, late-stage AMD and wet, or neovascular, AMD as well as combinations thereof [2, 3]. Neovascular AMD (nAMD) occurs in about two-thirds of late-stage AMD cases, develops rapidly, and commonly results in severe vision loss if left untreated.
Current pharmacologic therapy for nAMD involves intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) agents. Anti-VEGF monotherapy is the preferred treatment for nAMD with prompt intervention limiting visual loss in 95% of patients with nAMD [4, 5]. Patients with nAMD demonstrate high individual variability in the need for anti-VEGF injections. Delay in initiating treatment as well as undertreatment caused by poor adherence and/or persistence leads to worsening treatment outcomes over time [6,7,8]. Differing treatment regimens have been shown to impact treatment adherence and persistence, with treat and extend or comparable regimens fairing best. Thus, further research on the comparison between existing treatment regimens for nAMD is necessary.
Brolucizumab is a humanized single-chain antibody fragment that inhibits all isoforms of VEGF-A. Owing to its small size, brolucizumab demonstrates good tissue penetration in the eye, reaching high concentrations in the retina [9]. In two pivotal phase III trials, HAWK and HARRIER, brolucizumab demonstrated non-inferiority to aflibercept in the primary endpoint of change in best-corrected visual acuity (BCVA) in the treatment of nAMD [10]. Moreover, among key secondary endpoints of disease activity, brolucizumab was superior to aflibercept. These include reduction in retinal thickness (central subfield thickness) as well as retinal fluid markers (subretinal and intraretinal fluids), which are anatomical outcomes used to determine treatment efficacy and injection frequency in clinical practice [10]. Brolucizumab has been associated with uncommon events of retinal vasculitis and retinal vascular occlusion in patients with nAMD [11].
HAWK and HARRIER provided insight into the relative efficacy and safety of brolucizumab versus aflibercept. However, indirect comparisons of brolucizumab with other anti-VEGFs are needed.
The objective of the present study was to conduct a network meta-analysis (NMA) comparing the efficacy and safety of brolucizumab to other approved and marketed anti-VEGF treatments in patients with nAMD. By combining direct and indirect evidence, NMA makes it possible to simultaneously compare multiple treatments that were not compared in head-to-head trials.
Methods
Search Strategy
A systematic literature review of studies comparing anti-VEGFs in nAMD was conducted using Embase, Medline, Medline-in-Process, and Cochrane Library from database inception to June 3, 2021. The search strategy included English-language publications on clinical trials that evaluated treatments for patients with nAMD. The following treatments were included in the search strategy: brolucizumab, ranibizumab, aflibercept, faricimab, pegaptanib, bevacizumab, photodynamic therapy with verteporfin, laser photocoagulation therapy, and macular surgeries. The NMA only included licensed anti-VEGF therapies. While faricimab was not licensed when we conducted the study, it was included because Roche had filed an application in the European Union, and submitted to the National Institute for Health and Care Excellence (NICE) [12, 13]. Faricimab has been recently approved by the US Food and Drug Administration (FDA) [14]. The search strategy was not restricted to specific outcomes, as this was evaluated during the screening phase of the literature review. The detailed search strategy is available in the Supplementary Material.
In addition, relevant abstracts from 2015 to 2021 were searched from the following congresses: American Society of Retina Specialist, The American Macular Degeneration Foundation, European Society of Retina Specialists, The Retina International World Congress of Ophthalmology, The Association for Research and Vision in Ophthalmology, American Academy of Ophthalmology, The Royal Australian and New Zealand College of Ophthalmologists, Asia-Australia Controversies in Ophthalmology, and The Royal College of Ophthalmologists. The US National Library of Medicine (Clinicaltrials.gov) and the EU Clinical Trials Register were also searched for any additional studies of interest. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Study Population and Selection Criteria
The selection criteria for the NMA are presented in Table 1. In terms of participants, adults with a primary diagnosis of nAMD were included. Studies where patients with polypoidal choroidal vasculopathy (PCV) made up more than 10% of the population were excluded, as patients with PCV present different demographic and clinical characteristics than the overall nAMD population. Randomized controlled trials (RCTs) of 44 weeks or longer, crossover RCTs, and open-label extension studies of RCTs were also considered. In terms of treatments, licensed doses of brolucizumab, ranibizumab, and aflibercept were included in the NMA. As faricimab was not yet licensed at the time of analysis, all doses and regimens in its clinical trials were considered. Acknowledging unlicensed bevacizumab is reimbursed in some countries under specific protocols, a sensitivity analysis including bevacizumab is reported in Supplementary Material. Each treatment was evaluated separately by its administration regimen, including if a loading phase was used. The efficacy outcomes of interest included mean change in BCVA, proportion of patients gaining at least 15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters, proportion of patients losing at least 15 ETDRS letters, and mean change in retinal thickness. The safety outcomes included discontinuation, serious adverse events (SAE), including intraocular inflammation, endophthalmitis, retinal detachment, retinal tear, retinal pigment epithelial tear, cataract, and stroke. Additionally, the mean number of injections was evaluated.
Study Screening
Titles and abstracts of all citations were screened by two independent reviewers (ND, AQ), and discrepancies resolved by a third independent reviewer (CM). The full texts of relevant publications were screened using the same process.
Data Extraction and Quality Assessment
Two independent researchers (ND, AQ) extracted data from the identified publications into a prepared data extraction form. A third independent reviewer (CM) performed a quality check of all extractions. The quality and risk of bias of each included study were assessed using the Cochrane Collaboration’s quality tool [15].
Statistical Analysis
Heterogeneity was assessed for each direct comparison using pairwise meta-analyses when at least two studies reported the same treatment comparison. The Cochran’s Q test was conducted and the I2 statistic was calculated; if the p value of the Cochrane’s Q test was less than 0.10 or the I2 statistic was greater than 50%, then heterogeneity was suspected [16]. An inconsistency assessment using the Bucher approach was conducted for each closed loop in the networks with at least two trials. For each outcome, the indirect comparison based on the Bucher approach was compared to a direct pairwise meta-analysis [17]. The difference of the point estimates obtained from indirect versus direct evidence was calculated and a z-score was obtained. If the p value was lower than 0.05, then inconsistency was considered to be present.
The NMAs were conducted using a Bayesian framework in accordance with NICE Decision Support Unit (DSU) guidelines, and also accepted by most health technology appraisal bodies [18, 19]. The estimates were calculated using Monte Carlo Markov chains (MCMCs). Two MCMCs were used with 20,000 iterations and a burn-in of 20,000 for fixed-effects models; for random-effects models, 100,000 iterations were used with a burn-in of 100,000. The model with the lowest deviance information criterion (DIC) was selected. If the DIC of the random-effects model was less than 3 units lower than that of the fixed-effects model, then the fixed-effects model was chosen.
For the continuous outcomes, mean change in BCVA and mean change in retinal thickness, the posterior mean change from baseline was calculated along with 95% credible intervals (95%Crl). For the categorical outcomes, patients losing or gaining at least 15 letters and discontinuation, the odds ratio was reported along with the 95%Crl. Finally, for serious adverse events and injection frequency, the pooled absolute treatment effects were calculated using inverse variance weighting and random-effect models [20]. Serious adverse events were pooled by treatment molecule while injection frequency was pooled by treatment regimen.
Results
Systematic Literature Review
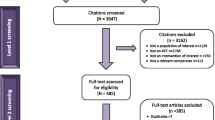
A total of 10,676 citations were captured from the electronic database searches. After removal of duplicates, 8826 citations remained. The screening of these titles and abstracts led to the review of 229 publications to assess their eligibility for inclusion. On the basis of the selection criteria, 65 publications were included, and their data was extracted in the narrative synthesis, as shown in the PRISMA diagram (Fig. 1). These publications encompassed 53 clinical trials. After applying the PICOS (Population, Intervention, Comparators, Outcomes Study design) criteria for inclusion in the NMA, a total of 19 trials were considered relevant for the NMA (Table 1). The details of the included trials are reported in Table 2, and the network for the primary outcome of interest, the mean change in BCVA, is represented in Fig. 2. For all other outcomes, the networks are reported in the Supplementary Material.
Network for mean change in BCVA at 1 year. Rani ranibizumab; Bro brolucizumab; Afli aflibercept; Fari faricimab; LP loading phase of 3 monthly injections, LP (4q4) loading phase of 4 monthly injections; PRN pro re nata or as needed; PRNX as needed with the potential to extend the treatment intervals; TREX treat and extend; q4, q8, q12, q16 injections administered every 4, 8, 12, and 16 weeks, respectively
The evidence provided by these 19 trials were overall of high quality. The greatest risk of bias was due to insufficient information identified in the publication during the quality assessment. Most studies did not report unexpected imbalances in dropouts between groups.
An additional ten trials were included in the sensitivity analyses with unlicensed bevacizumab. The details of the included trials are reported in Supplementary Material.
Characteristics and Comparability of Included Studies
Similar eligibility criteria were observed amongst the included trials. Nearly all trials included treatment-naive patients only, with the exception of MARINA and PIER. While MARINA and PIER did not report any inclusion of exclusion criteria related to prior anti-VEGF therapy, these trials were conducted when pegaptanib was the only authorized anti-VEGF agent. Given the lack of other authorized anti-VEGF treatments at the time, patients enrolled in these trials were likely to be predominantly treatment naive.
The average age at baseline varied between 65 years (TREX-AMD: LP → Rani 0.5TREX) and 81 years (RABIMO: LP → Rani 0.5PRN), with most trials reporting a mean age between 75 and 78 years. The proportion of male patients ranged from 30% (RABIMO: LP → Rani 0.5q8) to 56% (SALUTE: LP → Rani 0.5PRN). The mean BCVA at baseline was generally higher amongst the more recent studies, but this did not vary considerably. The lowest mean BCVA at baseline was reported for the sham injection arm of MARINA, at 53.6 ETDRS letters. The highest mean BCVA at baseline was reported for the LP → Rani 0.5TREX arm of RIVAL, at 65.3 letters.
In the included studies there were 11 different anti-VEGF regimens considered: injections administered on a fixed schedule every 4, 8, 12, or 16 weeks (q4, q8, q12, and q16), treatment administered as needed (PRN), PRN with the potential to extend the assessment interval (PRNX), treat with the potential to extend the treatment interval when no signs of exudation are present (TREX), injections every 8 weeks until week 40 then every 12 weeks until week 56 (q8 → q12), injections every 12 weeks unless there were signs of disease progression in which case the patient switched to bimonthly injections (q12/q8), and injections every 8 or 16 weeks on the basis of disease activity and assessment at weeks 20 and 24 (q8–q16). Some of the regimens included a loading phase of three initial monthly injections (LP). The faricimab trials, however, included a loading phase of four initial monthly injections (LP (4q4)).
Network Meta-Analysis
On the basis of convergence and DIC values, the fixed-effects model was chosen for each outcome. Among the closed loops, no statistical evidence of inconsistency was identified (Supplementary Material). The treatment comparisons for which significant heterogeneity was identified for mean change in BCVA at 1 year were LP → Rani 0.5TREX versus Rani 0.5q4, Rani 0.5q4 versus Afli 2q4, and LP → Afli 2q8 vs. Afli 2q4. In the first comparison, baseline characteristics were assessed and were similar across the three trials. This heterogeneity is therefore likely due to the inherent variability of the follow-up treatment intervals in the TREX regimen and cannot be controlled for. The second two comparisons were between similarly designed trials, VIEW 1 and VIEW 2, which used the same inclusion and exclusion criteria. The main difference between these studies was the geographical area considered, suggesting that the heterogeneity in results is due to random variability. These trials were therefore pooled in the NMA and considered one trial. This is the same approach that NICE used in their NMA in nAMD [21]. Detailed information from the heterogeneity assessment can be found in the Supplementary Material.
The relative efficacy and safety results for the brolucizumab 6 mg arm evaluated in HAWK and HARRIER (LP → Bro 6q12/q8) for each of the NMAs conducted at 1 and 2 years are presented in Figs. 3 and 4, respectively. The surface under the cumulative ranking curve (SUCRA) and probability for LP → Bro 6q12/q8 to perform better than each of its comparators are reported in Table 3. Compared to the sham injection, LP → Bro 6q12/q8 had a higher mean change in BCVA at both 1 and 2 years. None of the other relative treatments had significant effects based on the 95%Crl. LP → Bro 6q12/q8 had greater than a 50% probability of performing better than seven out of 16 of its comparators in terms of increasing BCVA at 1 year.
Forest plot of results obtained through the network meta-analysis for a mean change in BCVA at 2 years, b mean change in BCVA from 1 to 2 years, c mean change in retinal thickness at 2 years, d patients losing at least 15 letters at 2 years, e patients gaining at least 15 letters at 2 years, f overall discontinuation at 2 years
Brolucizumab 6 mg also had a significantly greater odds of gaining at least 15 letters than sham injections and a high probability of performing better than 12 out of 14 and eight out of nine of its comparators at 1 and 2 years, respectively. Additionally, LP → Bro 6q12/q8 had the third highest SUCRA for patients gaining at least 15 letters at 1 year, with a value of 73.7%. Similar results were obtained for the odds of losing at least 15 letters, with LP → Bro 6q12/q8 having a greater than 50% probability of performing better than 12 out of 15 of its competitors at 1 year.
A significantly greater decrease in mean retinal thickness was observed for brolucizumab 6 mg when compared to most of the competitors at both 1 and 2 years (10 out of 13 at 1 year, and 5 out of 8 at 2 years), with LP → Bro 6q12/q8 having the highest mean rank in SUCRA value at 1 year with 96.6%. Each treatment had similar odds of discontinuation, with no significant differences in relative treatment effects based on the 95%Crl.
Adverse Events and Injection Frequency
This NMA includes SAEs as reported in the original pivotal trials. Information for faricimab is limited at this stage, and isolated trial results do not reflect the latest brolucizumab safety information available. Results from the molecule-based pooled absolute treatment are briefly reported below, and fully included in the Supplementary Material for completion.
Endophthalmitis rates ranged from 0.31% for brolucizumab to 0.50% for ranibizumab at 1 year and from 0.54% for aflibercept to 0.94% for ranibizumab at 2 years. All anti-VEGF treatments reported similar rates of retinal detachment at 1 year, 0.30%, and ranging between 0.30% and 0.49% at 2 years. Rates of retinal pigment epithelial tear ranged between 0.30% and 1.10% at 1 year and between 0.20% and 0.40% at 2 years. Retinal tear was reported among 0.30% of patients receiving ranibizumab and none receiving aflibercept at 1 year. At 2 years, these rates ranged between 0.30% and 0.50%. Finally, at 1 year, stroke was reported by between 0.42% and 0.63% of patients; at 2 years, these rates ranged between 0.63% and 1.04%.
The results for the injection frequency at 1 year indicated that Afli 2q4 had the highest injection frequency and LP → Rani 0.5PRNX had the lowest. At 2 years, Rani 0.5q4 presented the highest injection frequency and the two brolucizumab regimens presented the lowest annualised injection frequency (Table 4). Fixed monthly injections are expected to have the highest mean number of injections based on their protocol for treatment administration.
Discussion and Conclusion
This study is the first of its kind in indirectly comparing the efficacy of brolucizumab and faricimab to all comparators for the treatment of nAMD. Across the board, the quality of evidence included in the NMA was high with mostly low bias risk. Among all anti-VEGF treatments, the visual acuity outcomes were similar, which is consistent with previous meta-analyses conducted in nAMD [21,22,23]. Many of the studies included ranibizumab and aflibercept as active comparators. However, ranibizumab is often administered on a monthly schedule, whereas newer treatments have less frequent or more flexible dosing regimens based on disease activity. In particular, when compared to the faricimab arm evaluated in TENAYA and LUCERNE at 1 year, brolucizumab had a lower number of injections, at 6.7 compared to 7.0. This is likely in part due to the loading phase consisting of intravitreal injections every 4 weeks for the first four doses required in the faricimab trials.
Brolucizumab also showed greater reductions in retinal thickness than its comparators. Retinal thickness is a common anatomical measure of disease activity in nAMD, and studies have shown that greater thickness may be associated with worse visual acuity outcomes [24, 25]. These measurements also play an important role in determining dosing intervals for variable dosing regimens. The results of the present study demonstrate that flexible dosing regimens of ranibizumab and aflibercept have similar efficacy on visual acuity as fixed dosing regimens while decreasing the disease burden for patients, caregivers, and physicians [10, 26, 27]. In addition, even with fewer injections, brolucizumab had significantly better morphological outcomes in terms of retinal thickness than its comparators (with comparable incidence of geographic atrophy and fibrosis across both treatment arms). However, further research is necessary to better understand the intricacies of the relationship between these anatomical outcomes and visual acuity.
The summary from our work on adverse events amongst anti-VEGF treatments in nAMD is in line with previous NMAs that evaluated ranibizumab and aflibercept [22, 28,29,30,31,32].
After the approval from the FDA, brolucizumab was associated with a safety signal of retinal vasculitis and/or retinal vascular occlusion (RV/RO), typically in the presence of intraocular inflammation [33]. These events were not reported with this wording, i.e., RV/RO, in the HAWK and HARRIER trials. The latest brolucizumab safety information can be found elsewhere, including in the brolucizumab.info website [11, 34,35,36,37,38,39].
RCTs, which are often considered the best available published evidence on comparative treatment efficacy and safety, have been shown to have low external validity and thus are limited when trying to decide which of multiple treatments is the most effective when used in clinical practice. This is in part due to the inclusion of only a highly select group of patients into RCTs, the lack of data on direct clinical comparisons, and the lack of clinically pertinent endpoints. In addition, a conventional pairwise meta-analysis cannot fully inform the comparisons between each treatment due in part to different populations being studied [40]. The application of an NMA helped overcome some of these limitations as all available evidence from the literature was pooled to indirectly compare treatments that had not been previously directly compared in a clinical trial. In particular, since only HAWK and HARRIER were head-to-head trials assessing the comparison between brolucizumab and aflibercept, conducting an NMA helped to estimate the relative efficacy of brolucizumab compared to other treatments not assessed in these two studies.
The process for conducting the present systematic literature review and NMA was based on reference standard guidelines, e.g., NICE [18, 41]. The results from this study were generally similar to the results of the NMA in nAMD conducted by NICE [21], and in the few differing cases the source of the discrepancy was found to be due to the decisions made on which studies to include. Patient characteristics among the included trials were similar at baseline, and little heterogeneity was identified in the direct comparisons, allowing for more robust results.
While the results from this NMA provide pertinent details comparing brolucizumab to its licensed comparators, several limitations were identified. First, it was not possible to conduct a meta-regression adjusted on relevant covariates such as baseline BCVA, age, and year of RCT start. The addition of these covariates yielded an over-parametrized model that could not converge. In addition, pooling indirect clinical evidence in an NMA does not necessarily reflect actual clinical practice, as the study population is selective in terms of inclusion and exclusion criteria, many included trials are historic, and patients must adhere to the study protocol. Further real-world studies evaluating these outcomes are therefore warranted. Moreover, patients with nAMD were evaluated in this study, and no specific subgroup analyses were conducted. Further research on the impact of anti-VEGFs on relevant subgroups, such as patients with PCV and types 1 (subpigment epithelial), 2 (subretinal), and 3 (retinal angiomatous proliferation) would provide a greater understanding of how these patient populations respond to the different treatments. Additionally, both patients naive to other anti-VEGF and mixed populations were included in the analyses. Only two trials, PIER and MARINA, did not report prior use of other anti-VEGF as an inclusion/exclusion criterion. However, patients included in these trials were likely to be treatment naive as they were pivotal ranibizumab trials that took place when pegaptanib was the only licensed anti-VEGF treatment. In order to include all available evidence for the treatments of interest, time equivalence was assumed between 48 and 52 weeks for 1-year outcomes and between 96 and 104 weeks for 2-year outcomes. Finally, an NMA for the mean number of injections was not conducted since the number of injections was directly related to the type of regimen specified in the study protocol. As such, the pooled absolute number of injections was estimated using robust methods. Similarly, while an NMA is a superior method to study relative effects for serious adverse events, it was not feasible because of serious adverse events being rare events and a general lack of reported data on individual adverse events.
On the basis of the results of the present study, brolucizumab has comparable BCVA gains and discontinuation rates compared to other licensed anti-VEGF treatments and is superior in terms of reducing retinal thickness to most treatments. Fewer injections were administered for brolucizumab than its other comparators to achieve these results during 2 years of treatment, while injection frequency was following the respective study protocol specifications.
References
Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1(4):314–21. https://doi.org/10.1016/j.oret.2016.12.004.
Chakravarthy U, Adamis AP, Cunningham ET, et al. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006;113(9):1508.e1-25. https://doi.org/10.1016/j.ophtha.2006.02.064.
Sepahi S, Mohajeri SA, Hosseini SM, et al. Effects of crocin on diabetic maculopathy: a placebo-controlled randomized clinical trial. Am J Ophthalmol. 2018;190:89–98. https://doi.org/10.1016/j.ajo.2018.03.007.
Cook HL, Patel PJ, Tufail A. Age-related macular degeneration: diagnosis and management. Br Med Bull. 2008;85:127–49. https://doi.org/10.1093/bmb/ldn012.
Mantel I. Optimizing the anti-VEGF treatment strategy for neovascular age-related macular degeneration: from clinical trials to real-life requirements. Transl Vis Sci Technol. 2015;4(3):6. https://doi.org/10.1167/tvst.4.3.6.
Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye (Lond). 2016;30(2):270–86. https://doi.org/10.1038/eye.2015.217.
Wolf A, Kampik A. Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol. 2014;252(4):647–55. https://doi.org/10.1007/s00417-013-2562-6.
Ziemssen F, Agostini H, Feltgen N, et al. A model to quantify the influence of treatment patterns and optimize outcomes in nAMD. Sci Rep. 2022;12(1):2789. https://doi.org/10.1038/s41598-022-06362-w.
Tadayoni R, Sararols L, Weissgerber G, Verma R, Clemens A, Holz FG. Brolucizumab: a newly developed anti-VEGF molecule for the treatment of neovascular age-related macular degeneration. Ophthalmologica. 2021;244(2):93–101. https://doi.org/10.1159/000513048.
Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. https://doi.org/10.1016/j.ophtha.2019.04.017.
Khoramnia R, Figueroa MS, Hattenbach LO, et al. Manifestations of intraocular inflammation over time in patients on brolucizumab for neovascular AMD. Graefes Arch Clin Exp Ophthalmol. 2021. https://doi.org/10.1007/s00417-021-05518-0.
European Medicines Agency, Committee for medicinal products for human use (CHMP), 2021. Draft agenda for the meeting on 21–24 June 2021. [Online] Available at: https://www.ema.europa.eu/en/documents/agenda/agenda-chmpagenda-21-24-june-2021-meeting_en.pdf. Accessed Jan 2022.
National Institute for Health and Care Excellence (NICE), 2021. Health Technology Appraisal, Faricimab for wet age-related macular degeneration, Draft Scope [Online] Available at: https://www.nice.org.uk/guidance/gid-ta10799/documents/draftscope-post-referral. Accessed Jan 2022.
VABYSMOTM (faricimab-svoa) injection, for intravitreal use. Accessed Feb 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761235s000lbl.pdf.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928. https://doi.org/10.1136/bmj.d5928.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. https://doi.org/10.1136/bmj.327.7414.557.
Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials. National Institute for Health and Care Excellence (NICE); 2014. Accessed Nov 11, 2021. http://www.ncbi.nlm.nih.gov/books/NBK310372/
Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. National Institute for Health and Care Excellence (NICE); 2014. Accessed Nov 11, 2021. http://www.ncbi.nlm.nih.gov/books/NBK310366/
Laws A, Kendall R, Hawkins N. A comparison of national guidelines for network meta-analysis. Value Health. 2014;17(5):642–54. https://doi.org/10.1016/j.jval.2014.06.001.
Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. https://doi.org/10.1002/jrsm.12.
National Institute for Health and Care Excellence (NICE), 2018. Macular degeneration, Appendix G: Network meta-analysis. [Online] Available at: https://www.nice.org.uk/guidance/ng82/evidence/appendix-gnetwork-metaanalysis-pdf-4723229204. Accessed Dec 2021.
Ye L, Jiaqi Z, Jianchao W, Zhaohui F, Liang Y, Xiaohui Z. Comparative efficacy and safety of anti-vascular endothelial growth factor regimens for neovascular age-related macular degeneration: systematic review and Bayesian network meta-analysis. Ther Adv Chronic Dis. 2020;11:2040622320953349. https://doi.org/10.1177/2040622320953349.
Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:CD005139. https://doi.org/10.1002/14651858.CD005139.pub4.
Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. https://doi.org/10.1016/j.ophtha.2020.06.028.
Evans RN, Reeves BC, Maguire MG, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti-vascular endothelial growth factor agents. JAMA Ophthalmol. 2020;138(10):1043–51. https://doi.org/10.1001/jamaophthalmol.2020.3001.
Silva R, Berta A, Larsen M, et al. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND study. Ophthalmology. 2018;125(1):57–65. https://doi.org/10.1016/j.ophtha.2017.07.014.
Kertes PJ, Galic IJ, Greve M, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology. 2019;126(6):841–8. https://doi.org/10.1016/j.ophtha.2019.01.013.
Zhao XY, Meng LH, Liu SZ, Chen YX. Efficacy and safety of different agents, dosages and strategies of anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration: a network meta-analysis of randomized controlled trials. Acta Ophthalmol. 2021;99(7):e1041–50. https://doi.org/10.1111/aos.14756.
Plyukhova AA, Budzinskaya MV, Starostin KM, et al. Comparative safety of bevacizumab, ranibizumab, and aflibercept for treatment of neovascular age-related macular degeneration (AMD): a systematic review and network meta-analysis of direct comparative studies. J Clin Med. 2020;9(5):E1522. https://doi.org/10.3390/jcm9051522.
Weber M, Velasque L, Coscas F, Faure C, Aubry I, Cohen SY. Effectiveness and safety of intravitreal aflibercept in patients with wet age-related macular degeneration treated in routine clinical practices across France: 12-month outcomes of the RAINBOW study. BMJ Open Ophthalmol. 2019;4(1): e000109. https://doi.org/10.1136/bmjophth-2017-000109.
Carrasco J, Daien V, Eldem BM, Spoorendonk JA, Yoon J. 2-year real-world outcomes with intravitreal aflibercept in neovascular age-related macular degeneration: literature review and meta-analysis of patient-relevant outcomes. Ophthalmol Ther. 2021;10(3):397–411. https://doi.org/10.1007/s40123-021-00350-5.
Holz FG, Figueroa MS, Bandello F, et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from LUMINOUS, a global real-world study. Retina Phila PA. 2020;40(9):1673–85. https://doi.org/10.1097/IAE.0000000000002670.
Novartis, 2020. Beovu [U.S. Prescribing Information]. [Online] Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761125s004lbl.pdf. Accessed Dec 2021.
Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion-related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128(7):1050–9. https://doi.org/10.1016/j.ophtha.2020.11.011.
Singer M, Albini TA, Seres A, et al. Clinical Characteristics and outcomes of eyes with intraocular inflammation after brolucizumab: post hoc analysis of HAWK and HARRIER. Ophthalmol Retina. 2021:S2468-6530(21)00162-7. https://doi.org/10.1016/j.oret.2021.05.003.
Baumal CR, Bodaghi B, Singer M, et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2021;5(6):519–27. https://doi.org/10.1016/j.oret.2020.09.020.
Holz FG, Heinz C, Wolf A, Hoerauf H, Pleyer U. Intraocular inflammation with brolucizumab use: patient management-diagnosis-therapy. Ophthalmologe. 2021;118(3):248–56. https://doi.org/10.1007/s00347-021-01321-8.
Sharma A, Kumar N, Parachuri N, et al. Brolucizumab-foreseeable workflow in the current scenario. Eye (Lond). 2021;35(6):1548–50. https://doi.org/10.1038/s41433-020-01324-w.
Novartis, 2022. Beovu [Global use and safety information for Healthcare Professionals]. [Online] Available at: https://www.brolucizumab.info/. Accessed Apr 2022.
National Institute for Health and Care Excellence, 2014. Network meta-analyses – methods and detailed results. [Online] Available at: https://www.nice.org.uk/guidance/cg184/evidence/appendix-enetwork-metaanalyses-methods-and-detailed-results-pdf-193203763. Accessed Dec 2021.
NICE Guide to the methods of technology appraisal. Published online 2013. http://www.nice.org.uk/article/pmg9/resources/non-guidance-guide-to-the-methods-of-technology-appraisal-2013-pdf
Acknowledgements
Carole Mamane (CM) is an employee of Amaris Consulting and contributed as a third independent reviewer in the systematic literature review.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by Novartis Pharma AG. Novartis Pharma AG is also funding the journal’s Rapid Service and Open Access Fees.
Medical Writing and Editorial Assistance
Medical writing and editorial assistance were provided by Natalie Dennis, Arthur Quenéchdu and Nicolas Bertin of Amaris. This was funded by Novartis Pharma AG.
Author Contributions
RPF and ES are medical experts who contributed to the design, interpretation of results and to the writing of the manuscript. RF, AC, HK led the concept and design, interpretation of results and writing of the manuscript. ND and AQ were in charge of the literature review, statistical analysis and writing of the manuscript.
Disclosures
Robert P. Finger is a consultant for Bayer, Novartis, Roche/Genentech, AbbVie/Allergan, Alimera, Böhringer-Ingelheim, Santhera, Ellex, ProQR, Opthea, Chiesi, ProQR and research supported for Novartis, Zeiss Meditec, Heidelberg Engineering, CentreVue, Biogen. Rita Freitas is a full-time employee and shareholder at Novartis Farma – Produtos Farmacêuticos S. A. Natalie Dennis and Arthur Quenéchdu are employees of Amaris Consulting. Andreas Clemens is a full-time employee and shareholder at Novartis Pharma AG. Helene Karcher is a full-time employee and shareholder at Novartis Pharma AG and Editor-in-Chief at Epidemiologic Methods. Eric H. Souied is an expert and board member for Roche, Novartis, Allergan/Abbvie, Bayer, Teva.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Finger, R.P., Dennis, N., Freitas, R. et al. Comparative Efficacy of Brolucizumab in the Treatment of Neovascular Age-Related Macular Degeneration: A Systematic Literature Review and Network Meta-Analysis. Adv Ther 39, 3425–3448 (2022). https://doi.org/10.1007/s12325-022-02193-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02193-3