Abstract
Introduction
Patients with advanced Parkinson’s disease (PD) may require device-aided therapies (DAT) for adequate symptom control. However, long-term, real-world efficacy and safety data are limited. This study aims to describe real-world, long-term treatment persistence for patients with PD treated with levodopa-carbidopa intestinal gel (LCIG). The study also aims to describe patient profiles, treatment discontinuation rates, co-medication patterns, monotherapy rates, and rates of healthcare visits and their associated costs for patients receiving all forms of DAT (deep brain stimulation [DBS], continuous subcutaneous apomorphine infusion [CSAI], or LCIG).
Methods
In this retrospective analysis of the Israeli Maccabi Healthcare Services database, adult patients with PD were analyzed in three cohorts, based on DAT (DBS, CSAI, or LCIG). The primary endpoint was LCIG treatment persistence 12 months after initiation.
Results
This analysis included 161 DAT-treated patients (LCIG, n = 62; DBS, n = 76; CSAI, n = 23). Among those who discontinued, the mean time to discontinuation was 86.4 months for LCIG and 42.4 months for CSAI (p = 0.046). Twelve months after initiation, 14.3% LCIG, 10.7% DBS, and 5.9% CSAI patients were not receiving any additional anti-parkinsonian therapy. At the last recorded visit, 28.6% LCIG, 13.3% DBS, and 5.9% CSAI patients received DAT as monotherapy. During the first 12 months after initiation, 45.2% LCIG, 65.2% CSAI, and 1.3% DBS patients had no reported hospitalization days. Annual healthcare visit costs decreased following LCIG initiation (US$9491 vs. $8146) and increased following DBS ($4113 vs. $7677) and CSAI ($6378 vs. $8277).
Conclusion
DAT are well maintained in patients with advanced PD. These retrospective data suggest that patients receiving LCIG may have higher long-term persistence rates compared with patients receiving CSAI. A subgroup of patients was treated with DAT as monotherapy without additional oral anti-parkinsonian therapy, with LCIG showing the highest rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is limited information on long-term, real-world use of device-aided therapies among patients with advanced Parkinson’s disease (PD). |
This study utilizes anonymized patient data from the Maccabi Healthcare Services database to examine real-world treatment persistence, co-medication, and healthcare visits and associated costs among patients with PD receiving deep brain stimulation (DBS), continuous subcutaneous apomorphine infusion (CSAI), or levodopa-carbidopa intestinal gel (LCIG). |
What was learned from this study? |
Device-aided therapies are well maintained among patients with advanced PD. |
Treatment with LCIG was associated with longer treatment persistence than treatment with CSAI, and LCIG was associated with higher rates of use as a monotherapy compared with CSAI or DBS. |
Healthcare visits and associated cost decreased after LCIG initiation but increased following DBS and CSAI. |
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by loss of dopaminergic neurons in the substantia nigra, resulting in the characteristic symptoms of bradykinesia, rigidity, and tremor [1]. In addition to motor symptoms, patients with PD often experience non-motor symptoms that include neuropsychiatric, sleep, autonomic, sensory, and gastrointestinal symptoms [2]. While oral levodopa is the gold standard for PD treatment, the therapeutic window of oral levodopa narrows with progressive disease, and patients may experience symptom fluctuation [1].
Device-aided therapies (DATs) may be required to achieve adequate symptom control in patients with advanced PD [3]. DAT that are available at present are continuous subcutaneous apomorphine infusion (CSAI), deep brain stimulation (DBS), and levodopa-carbidopa intestinal gel (LCIG) [3, 4]. Using DAT as a monotherapy may help to minimize drug–drug interactions, promote better treatment adherence, and decrease subjective medication burden [5,6,7]. However, despite the demonstrated effectiveness of these treatments, patients usually require supplemental treatment with oral anti-parkinsonian therapy while they are receiving DAT [3, 8, 9].
LCIG delivers a continuous intestinal infusion of levodopa-carbidopa, allowing for more stable plasma levels of levodopa relative to oral levodopa therapy [10, 11]. Results from post hoc analyses of the GLORIA (Global LOng-term Registry on efficacy and safety of LCIG In patients with Advanced Parkinson’s disease in routine care) and COSMOS (COmedication Study assessing Mono- and cOmbination therapy with levodopa-carbidopa inteStinal gel) studies have demonstrated that LCIG could be used as monotherapy for the control of motor symptoms, with almost a quarter of patients in routine clinical care continuing long-term monotherapy for 2 years [7, 12]. CSAI is administered subcutaneously via a fine-caliber tube from an infusion pump. Patients often receive CSAI in conjunction with oral medications, although CSAI may be given as a monotherapy [3, 9]. DBS involves the surgical implantation of electrodes in specific regions of the brain, most commonly the subthalamic nucleus or the globus pallidus [13]. DBS is typically used in conjunction with comedications, with the goal of balancing oral medication with stimulation effects [3].
Databases of electronic healthcare records are useful tools to study the real-world outcomes that can be achieved with these treatments. In this study, we examine long-term patient data from the Israeli Maccabi Health Services (MHS) to investigate real-world DAT treatment for patients with advanced PD. This analysis characterizes multiple aspects of DAT use for advanced PD in a real-world setting. The primary goal of this retrospective analysis of the MHS database is to describe the real-world, long-term treatment persistence with LCIG as an indirect indication of a balance between efficacy and safety that is acceptable to patients. For this study, treatment persistence is measured based on the time to treatment discontinuation, and the associated probability of treatment discontinuation after 12 months. The study also aims to describe the profiles of patients treated with each DAT, assess discontinuation rates, therapy duration, and healthcare visits (outpatient, emergency department, and hospitalizations) and the costs associated with these visits for patients using DATs, and evaluate co-medication patterns and monotherapy rates across the population of patients with advanced PD who are treated with DATs.
Methods
Study Design
MHS is the second largest health maintenance organization in Israel, and maintains a database that includes the electronic medical records of approximately 2.5 million active members (approximately 25% of the population of Israel) dating back to 1993. This “cradle-to-grave” database provides physicians and researchers with long-term follow-up information on member patients because of the program’s high retention rate, with < 1% attrition annually. The database is representative of the Israeli population, as it comprises data from an even distribution of clinics and service providers throughout Israel, a country with universal healthcare. MHS collects data from medication prescriptions, MHS pharmacy network purchases, the MHS central laboratory, consultations, hospitalizations, and procedures.
We performed a retrospective analysis of the MHS database from 1 September 2009, when LCIG first became available in Israel (DBS and CSAI were already available at this time), through the cutoff date of 28 February 2019. The study was approved by the Bayit balev internal review board committee of MHS (reference number 0057-18-BBL), and patient data were anonymized and de-identified before undergoing analysis. Bayit balev of the Maccabi Group is the committee of the MHS and approves Maccabi's studies, including the research use of Maccabi's databases. Patients were separated into three cohorts based on DAT therapy (DBS, CSAI, or LCIG). Data were collected on prescription dispensing (including drug dosing), comorbidities, healthcare visits, employment status, use of assisted ambulatory devices (e.g., wheelchair or walker), caregiver support, and average duration of therapy.
Patients
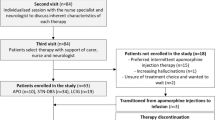
This analysis included all patients in the MHS database who initiated DAT therapy since 1 September 2009, were aged 18 years or older, and had a diagnosis of PD based on International Statistical Classification of Diseases and Related Health Problems, 9th revision (ICD-9; currently used by the healthcare system of Israel) coding (Fig. 1). By using the ICD-9 diagnosis of idiopathic PD as an inclusion criterion, secondary and atypical Parkinson syndromes were automatically excluded, as these syndromes have different ICD-9 codes. Additionally, by focusing on patients receiving DAT therapy, it is also highly likely that patients diagnosed with other extra-pyramidal syndromes (including atypical or secondary parkinsonism) were not included in this analysis, as use of DAT in these patients is rare. This analysis focused on patients receiving DAT, rather than those whose PD was considered advanced. Patients early in the course of PD would not be included based on this criterion, as these patients are managed with oral therapies. Patients who started DAT treatment < 12 months before the cutoff date, regardless of whether they had discontinued therapy, were excluded. To reflect the real-world population with advanced PD, patients with other comorbidities, including cancer, were not excluded from this analysis, as patients with advanced PD often have disease-related and non-disease-related comorbidities.
Reasons for exclusion. aPatients were excluded for not having a diagnosis of PD. The diagnoses of these patients were not recorded in this study. CSAI continuous subcutaneous apomorphine infusion, DAT device-aided therapy, DBS deep brain stimulation, LCIG levodopa-carbidopa intestinal gel, PD Parkinson’s disease
Assessments
Data were collected at baseline (before initiation of DAT), 12 months after initiation, and the last documented visit before treatment discontinuation or the cut-off date on 28 February 2019. The primary endpoint for the analysis was treatment persistence at 12 months following initiation of LCIG therapy, as assessed by the estimated rate of subjects continuing treatment. Secondary endpoints included 12-month treatment persistence for CSAI, long-term treatment duration with LCIG or CSAI, and treatment persistence at months 12, 24, 36, 48, and 60 with LCIG or CSAI. Continuation of DBS could not be measured, as the device is not removed, so discontinuation is not reflected in the dataset. The treatment discontinuation date was calculated as the last date medications were purchased together with the number of days medication was supplied; if patients died before the end of the period in which medication was supplied, they were censored rather than counted as discontinued.
Secondary endpoints for all patients using DAT included the proportion of patients using DAT as monotherapy, the proportion of patients taking 1, 2, 3, or 4 or more co-medications at 12 months, the last documented visit with ongoing DAT, the additional levodopa equivalent daily dose (LEDD) at 12 months and last documented visit, and healthcare resource consumption (including hospitalization days, emergency department visits, outpatient visits, and healthcare visit cost per year) 12 months before initiation of DAT, 12 months after initiation of DAT, and for 12 months before the last documented visit with ongoing DAT. Safety was measured via free text searches for adverse events (AEs). LEDD did not include levodopa administered via LCIG or apomorphine administered via CSAI, as it was not possible to determine how much dopa or apomorphine was administered by these routes because only prescription data were available. Two models of monotherapy were used for this analysis: a stringent definition in which patients received only DAT (model 1), and a broader definition, which allowed patients to also receive the controlled-release formulation of oral levodopa in addition to DAT (model 2). Some data, including patient characteristics and the occurrence of AEs, were extracted from patient records from the review of physicians’ notes.
Statistical Analysis
Results are presented using descriptive statistics. Baseline characteristics were compared using the chi-square test for categorical variables and one-way analysis of variance or the Kruskal–Wallis test for continuous variables, depending on the variable distribution. Baseline healthcare visit costs were compared using a generalized linear model with gamma distribution with log-link. The Kaplan–Meier method was used to estimate the DAT discontinuation rates and the 95% confidence interval (CI), and comparisons between cohorts were conducted using the log-rank test.
Healthcare visit costs are presented as annualized data. Unit costs were extracted from the Israeli Ministry of Health price list and converted to US dollars using purchasing power parities 2018 ($1 USD =  3.663 Israeli shekels) [14]. The economic analysis was focused on healthcare resource use in terms of direct medical costs. Healthcare visit costs consist of hospital, emergency department, and outpatient visits. Medications (including PD-related medications and DAT) were not included in healthcare visit costs.
3.663 Israeli shekels) [14]. The economic analysis was focused on healthcare resource use in terms of direct medical costs. Healthcare visit costs consist of hospital, emergency department, and outpatient visits. Medications (including PD-related medications and DAT) were not included in healthcare visit costs.
Healthcare visits (categorical variables) were compared between therapies for each time point using the chi-square test. Healthcare visit costs were compared between therapies using generalized linear mixed models with gamma distribution with log-link. As there was at least one outpatient visit per patient, there was no need to account for zero costs. No adjusted analyses were performed. The three time frames (the 12 months before DAT, the 12 months following DAT, and the 12 months before the last DAT measurement) were addressed as repeated measurements.
Results
Patients
A total of 591 patients receiving DAT were identified (LCIG, n = 77; DBS, n = 373; CSAI, n = 141). After applying the study exclusion criteria, 430 patients were excluded and 161 eligible patients were included in the analysis (LCIG, n = 62; DBS, n = 76; CSAI, n = 23; Fig. 1). Overall, baseline demographics were generally similar between groups (Table 1; Supplementary Table S1). However, patients with DBS tended to be younger at initiation of DAT (p = 0.003) and had a slightly shorter disease duration before receiving DBS than did patients who received LCIG or CSAI (p = 0.002). While median baseline daily LEDD was slightly lower in patients who were treated with DBS (1175 mg for DBS vs. 1667 mg and 1475 mg for LCIG and CSAI, respectively; p = 0.011), median daily levodopa tablet frequency was similar in all groups (5–6 per day; p = 0.397). Daily LEDD does not include levodopa delivered via LCIG or apomorphine administered via CSAI, as the dose could not be estimated from the prescription data. Hypertension was the most common comorbidity in all groups. Caregiver support from foreign workers was reported by most patients receiving caregiver support for all groups.
Treatment Persistence and Use as a Monotherapy
Twelve months following initiation of LCIG and CSAI, the median time-to-treatment discontinuation had not been reached (Fig. 2; Supplementary Table S2); after 12 months, 89.3% of patients who received LCIG and 82.4% who received CSAI were still receiving treatment. During the full 9.5-year follow-up period, among those who discontinued treatment, the mean time to discontinuation was 86.4 (95% CI, 73.3 – 99.6) months for LCIG and 42.4 (95% CI, 27.7 – 57.1) months for CSAI (p = 0.046; Fig. 2). Note that the mean times are presented here, as the median time was not met for the LCIG group; the median time to discontinuation of CSAI was 34.6 months.
Time to discontinuation of DAT. p values were calculated using the log-rank test. CSAI continuous subcutaneous apomorphine infusion, DAT device-aided therapy, LCIG levodopa-carbidopa intestinal gel. aMean months of follow-up are shown as median not reached in LCIG group. p value was calculated using log-rank test
Among patients with at least 12 months’ follow-up, the proportion of patients using DAT as monotherapy at 12 months [defined as no other additional anti-parkinsonian medications including oral levodopa (model 1)] was 8/56 (14.3%) for patients receiving LCIG, 8/75 (10.7%) for patients receiving DBS, and 1/17 (5.9%) for patients receiving CSAI (Fig. 3). This pattern was maintained during the last visit with DAT, with 16/56 (28.6%) of patients receiving LCIG, 10/75 (13.3%) receiving DBS, and 1/17 (5.9%) receiving CSAI continuing monotherapy (model 1) treatment. Similarly, at 12 months of DAT use, a greater proportion of patients receiving LCIG were using only one anti-parkinsonian medication (including but not limited to oral levodopa) in addition to DAT than were using two, three, or four or more drugs (one treatment, 39.3%; two treatments, 19.6%; three treatments, 14.3%; four or more treatments, 12.5%; p < 0.001). More than half of patients receiving CSAI used four or more additional PD treatments at all time points.
Treatments for Parkinson’s disease given in addition to DAT over time. CR controlled-release, CSAI continuous subcutaneous apomorphine infusion, DAT device-aided therapy, DBS deep brain stimulation, LCIG levodopa-carbidopa intestinal gel, PD Parkinson’s disease. aZero added PD medications represents monotherapy. bPatients with DAT monotherapy and only CR formulations of levodopa as additional oral co-medication. cNumber of added PD medications refers to those given in addition to DAT in the 6 months before baseline, 90 days before and 90 days after the 12-month timepoint, and 6 months before final DAT. Levodopa may be included in the number of added PD medications. p values were calculated using chi-square test
In the 12 months after the initiation of DAT and at the last measurement of DAT, the mean daily LEDD of added medications (i.e., non-DAT medications) was significantly lower in the groups receiving LCIG and DBS, with a slight increase in the group receiving CSAI (LCIG vs. CSAI, p < 0.001; DBS vs. CSAI, p < 0.001; Supplementary Fig. S1).
Reports of AEs as extracted from the review of notes in patient records are summarized in Supplementary Table S3. The most mentioned AE was redness/infections around the implant [LCIG, 14 (22.6%); DBS, 4 (5.3%); CSAI, 0 (0%)]. There were 37 deaths; LCIG, 22 (35.5%); DBS, 6 (7.9%); CSAI, 9 (39.1%). Median (IQR) age at death was: 75.5 (69.8 − 80.6) years LCIG; 80 (74.4 − 80.1) years DBS; 82.6 (76.2 − 85.7) years CSAI.
Healthcare Visits
Patients receiving LCIG demonstrated a trend toward a reduced number of hospitalization days (p < 0.001), with a higher proportion of patients having no hospitalization days in the 12 months after the initiation of treatment than before (45.2% vs. 1.6%; Supplementary Fig. S2). Hospitalizations for the 12 months leading to the last LCIG measurement were consistent with those for the 12 months following LCIG initiation (0 hospitalization days, 48.4% vs. 45.2%). Patients treated with DBS (p < 0.001) had more hospitalization days in the 12 months following treatment initiation compared with the 12 months prior to treatment (1.3% vs. 68.4%). The largest increase in hospitalization days among patients treated with DBS was observed in the group having 5–9 days of hospital stays in a year. In the last DAT measurement, however, hospitalizations among patients with DBS were similar to baseline levels (5–9 hospitalization days, 3.9% vs. 5.3%). Slightly fewer patients treated with CSAI (p = 0.192) had no hospitalization days in the 12 months after treatment initiation than before (65.2% vs. 60.9%). In the last DAT measurement, the proportion of patients with 0 hospitalization days decreased (43.5%), while the proportion of patients with 1–4 (30.4%) or ≥ 15 (21.7%) hospitalization days increased.
The trends in emergency department visits were consistent across the three treatments, with a slight increase in the proportion of patients having two or more emergency department visits (p > 0.05 for all). While this increase was modest for patients treated with LCIG and DBS, it was substantial for those treated with CSAI (17.4% 12 months after treatment initiation vs. 0% before treatment). At the last DAT measurement, emergency department visits were unchanged from 12 months following DAT initiation for patients receiving CSAI, were similar to pre-DAT levels for patients receiving DBS, and, for patients receiving LCIG, more patients had either 0 or ≥ 2 emergency department visits than at prior timepoints. For outpatient visits, the trends among patients receiving LCIG (p = 0.95) were fairly consistent before and after initiation of treatment. However, there was an increase (p < 0.01 for all) in the proportion of patients having > 40 outpatient visits 12 months after initiation of treatment versus before initiation of treatment in the DBS (61.8% vs. 43.4%) and CSAI (91.3% vs. 78.2%) groups. Outpatient visits were generally similar between baseline and last DAT measurement for LCIG and DBS, and were similar between 12 months post-DAT and last DAT measurement for CSAI.
In the 12 months before initiating DAT (i.e., baseline period), median annualized healthcare visit costs were almost 50% higher in the group of patients receiving LCIG ($9491) than in the group receiving CSAI ($6378), and more than twice that of the group receiving DBS ($4113; Supplementary Table S4). For patients receiving LCIG, healthcare visit costs decreased in the first 12 months of DAT use to $8146, while healthcare visit costs increased in the first 12 months for patients receiving DBS and CSAI. Beyond the first 12 months, patients treated with LCIG and DBS demonstrated reduced healthcare visit costs in the 12 months before the last DAT measurement compared with the 12 months after treatment initiation (LCIG, $5778 vs. $8146; DBS, $4320 vs. $7677). Healthcare visit costs rose slightly for those treated with CSAI in the 12 months before the last DAT measurement compared with the 12 months after treatment initiation ($8696 vs. $8277).
Discussion
In this retrospective, non-confirmatory analysis, long-term persistence rates with LCIG therapy are high (89.3% after 12 months), with a mean time to discontinuation of 86.4 months, while CSAI had a lower long-term persistence rate and a shorter time to discontinuation. Continuation with DBS cannot be readily measured in the MHS database because the device is not typically removed after it ceases to offer benefit. Therefore, DBS is not included in the analysis of treatment persistence, but is included in comedication and healthcare visit analyses to provide real-world information for all available forms of DAT.
To our knowledge, this is the first long-term, real-world assessment of treatment persistence on DAT with data that cover a study period of nearly 10 years. The discontinuation rate for LCIG observed in our study was 10.7% in the first 12 months. This compares favorably with results from a 12-month phase 3 clinical trial in which 23.2% of patients discontinued before the end of the trial [15]. Patients enrolled in this study and another phase 3 study were able to continue with LCIG in a rollover study until they transitioned to a commercially available product; overall, 75% of patients continued LCIG for at least 3 years (with a mean LCIG exposure of 4.1 years) [16]. In the DAPHNE study, 36% of patients treated with LCIG discontinued therapy during 3 years of follow-up [17]. A post hoc analysis of the GLORIA registry found a 12-month discontinuation rate of 17% for stable LCIG monotherapy and 23% for stable LCIG-based polytherapy [7]. In a real-world assessment of patients receiving CSAI, 34% discontinued by month 24, with a mean treatment duration of 7.4 months [18]. In contrast, patients in our study receiving CSAI had a 24-month continuation rate of 53.8% and a mean treatment duration of 42.4 months. Finally, results from a recent international prospective, multicenter, real-life cohort observational study (EuroInf 2), which also included patients whose PD symptoms were managed with DBS, showed similar results, with no patients discontinuing any treatment during the 6-month study [19]. Our analysis extends the reported duration of LCIG treatment in the real world, with a longer follow-up period than that in the GLORIA registry and COSMOS studies (2 years prospectively and a mean of 3 years retrospectively). While our analysis includes a smaller total population than that of the GLORIA or COSMOS studies, the Maccabi database comprises data from approximately 25% of the population of Israel. Therefore, patients included here represent approximately 25% of the total number of patients using DAT for PD treatment in Israel. Our results suggest that LCIG can be effective for long-term management of advanced PD in most patients [7, 12].
Patients receiving LCIG have a higher rate of monotherapy use than do patients who receive DBS or CSAI. While these data do not allow for speculation on the reasons for the differences in monotherapy rates, treatment patterns observed here reflect the common practice of avoiding the discontinuations of all other anti-parkinsonian medications while using DBS or CSAI. These results are consistent with those from a recent study that found that 26.8% of patients were managed with LCIG monotherapy after 6 months, compared with 7.6% treated with DBS [20]. Patients taking LCIG also had a reduction in the total number of drugs used to treat PD, with little change seen with patients taking CSAI or DBS. LCIG monotherapy has also been shown to have a similar safety profile to polytherapy, giving confidence that a simplified treatment approach is an appropriate option for patients with advanced PD [21].
There have been few long-term, real-world analyses of DAT use in patients with advanced PD. Furthermore, there is a lack of data on the ability of these therapies to be relied on as monotherapy in this setting. Similar to results observed in this study, findings reported in the COSMOS study revealed that patients who received LCIG as monotherapy showed similar symptom control to those patients who were taking LCIG in combination with other PD medications [12]. This was also confirmed in a post hoc analysis of the GLORIA registry [7]. Of note, patients in this study were taking a median of five to six levodopa tablets daily, providing support for the 5-2-1 criteria for identification of patients with advanced PD [22, 23].
It is important to evaluate healthcare use as advancing PD is associated with significant economic burden that impacts patients, caregivers, and healthcare systems [24]. Initiation of DAT requires comprehensive evaluation and follow-up, with some devices requiring surgical interventions [9]. Evaluating the frequency of healthcare visits and their associated costs after DAT initiation is important to quantify one aspect of the economic value of DAT and inform treatment selection and coverage. In this study, hospitalization days decreased in the 12 months following LCIG initiation compared with the 12 months prior to initiation, while for DBS, hospitalization days increased. While we have not studied the underlying causes of the differences in hospitalization days in the three groups, they are likely attributable to underlying disease severity at baseline, patient demographics, clinical characteristics, and the treatment modalities themselves. Reasons for healthcare visits (including hospitalizations, emergency department visits, and outpatient visits) were not captured, thus limiting our ability to discern between an AE-related visit versus a PD-related visit versus a non-PD–related visit.
A major advantage of MHS is that it provides patient data that spans multiple decades with a low attrition rate (< 1% annually), and the use of electronic records helps to facilitate data analysis and processing. The fact that patients remain in the database long term allows for a “cradle-to-grave” analysis of patients’ diseases, and makes it one of the most comprehensive databases available. MHS is based in Israel, a country with universal healthcare, and where citizens can choose their health maintenance organization and are able to switch from one plan to another. Another benefit of this healthcare database is that data can be extracted from free text searches of patients’ records. Some of the data in this analysis, including AEs, were derived from manual text searches of physicians’ notes attached to the electronic medical record. However, these data are limited by the level of detail provided in the notes. As a result, any variability in the completeness of these notes may influence the reported results, especially in the smaller groups. Additional potential indicators of safety, such as prescription data, were not used in this analysis, as indirect inference of AEs from prescriptions would be highly speculative.
Another limitation was the relatively small size of the population, notably the 23 patients included in the group receiving CSAI. As a result, it was not possible to match patients in the three different groups, so no adjusted analyses were conducted. In addition, a selection bias for the prescription of the different treatments at baseline, based on demographic and clinical characteristics such as PD severity, cannot be ruled out. In particular, patients receiving DBS were younger, with a shorter disease duration at baseline than patients receiving LCIG and CSAI. As a result, this study should be regarded as hypothesis generating rather than hypothesis testing. However, given the lack of real-world data on the use of DAT to treat advanced PD, this analysis provides important insights into the use of DAT in the clinical setting and should help to inform its role moving forward.
In this analysis, treatment persistence was used as an indirect indication of efficacy and safety of DAT. A lack of true clinical measures of disease state and severity (e.g., “Off” and “On” dyskinesia times, scores on clinical scales like the Unified Parkinson's Disease Rating Scale or Non-Motor Symptom Scale) are a key weakness of this study. In the EuroInf study, both CSAI and LCIG therapies had beneficial effects on motor and non-motor symptoms, as well as health-related quality of life, indicating that these treatments are effective in a real-world setting, although follow-up was limited to 6 months [25]. Continuation of therapy in our analysis does suggest that the risk–benefit calculation led to continuation of treatment, and hence continued effectiveness can be assumed. Although it was not possible to measure continuation of DBS in this analysis, the fact that follow-up of treatment with DAT in a real-world setting for a mean of approximately 7 years for treatment with LCIG and 3.5 years for CSAI suggests that these devices can have long-term efficacy. Indeed, results of studies in other therapeutic areas have shown a correlation between continuation of therapy and clinical efficacy [26]. It should be noted, however, that data for the last visit are for the 12 months before that visit, so these data are based on variable length of follow-up, potentially skewing the results in some cases. As PD is a progressive disease, this can bias long-term results in favor of therapies with a shorter follow-up. Therefore, definitive conclusions cannot be drawn from the last visit data, particularly regarding comedication and healthcare visits. Despite this methodological weakness, this approach does allow for a long natural follow-up in a real-world setting.
Reasons for patient discontinuation of DAT in this analysis (e.g., AEs, lack of effectiveness) are not available. However, a retrospective examination of medical records for patients receiving CSAI for at least 6 months found that the most common reason for discontinuation was troublesome dyskinesia, and many younger patients transitioned to DBS following CSAI therapy [27]. A recent retrospective analysis of patients who received LCIG for an average of 2.6 years found that the most common reasons for discontinuation were device-related side effects and less efficacy than expected by the patient [28]. In another retrospective study, the discontinuation rate was 21% during an average of 22 months of LCIG treatment, with death and poor compliance being the primary reasons; device-related complications and complications with percutaneous endoscopic gastro-jejunostomy occurred, but did not lead to the discontinuation of LCIG [29].
There were also some limitations to the healthcare visit analysis. The costs reported here reflect the costs of hospitalizations, emergency department visits, and outpatient visits, and do not include the costs of medications, indirect costs (i.e., loss of productivity) nor the cost of DAT. In the first 12 months of DAT use, most patients receiving DBS experienced one to nine hospitalization days, more than in the other two groups of participants. There is no single protocol for follow-up of patients receiving DBS in Israel. While each center has different regulations, none begin adjusting DBS immediately after surgery. In this analysis, hospitalization days in the first 12 months of DAT use include visits for DAT placement and adjustment, including an (optional) nasojejunal test phase for LCIG. Differences in hospitalization days likely reflect the invasive nature of device placement and the need for its adjustment rather than any negative sequelae. It is not possible to distinguish reasons for hospitalization in this dataset, limiting our ability to reach conclusions about healthcare visits and their associated costs. However, these results do agree with previous data indicating treatment with LCIG may stabilize long-term healthcare costs [17]. It is also not possible to accurately estimate LEDD for patients receiving LCIG or CSAI because the levodopa/apomorphine dose administered via the respective devices cannot be accurately estimated from prescription data alone. Future analyses may explore the impact of DAT on other economic outcomes such as indirect and medication costs.
Conclusions
DAT are effective and well maintained in patients with advanced PD. Results from our retrospective analysis of the MHS database suggest that patients receiving LCIG treatment may have higher long-term persistence rates compared with patients receiving CSAI. A subgroup of patients was treated with DAT as a monotherapy without any additional oral anti-parkinsonian medications. Patients treated with LCIG had the highest rates of DAT monotherapy (up to 29% in the long-term follow-up). Healthcare visits decrease after initiation of LCIG, as reflected by a low number of hospitalization days and reduced overall healthcare visit costs.
References
Antonini A, Chaudhuri KR, Martinez-Martin P, Odin P. Oral and infusion levodopa-based strategies for managing motor complications in patients with Parkinson’s disease. CNS Drugs. 2010;24(2):119–29.
Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464–74.
Odin P, Ray Chaudhuri K, Slevin JT, Volkmann J, Dietrichs E, Martinez-Martin P, et al. Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Parkinsonism Relat Disord. 2015;21(10):1133–44.
Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, et al. International Parkinson and Movement Disorder Society evidence-based medicine review: update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248–66.
DeMaagd G, Philip A. Parkinson’s disease and its management: part 3: Nondopaminergic and nonpharmacological treatment options. P T. 2015;40(10):668–79.
Fleisher JE, Stern MB. Medication nonadherence in Parkinson’s disease. Curr Neurol Neurosci Rep. 2013;13(10):382.
Poewe W, Bergmann L, Kukreja P, Robieson WZ, Antonini A. Levodopa-carbidopa intestinal gel monotherapy: GLORIA registry demographics, efficacy, and safety. J Parkinsons Dis. 2019;9(3):531–41.
Antonini A, Isaias IU, Rodolfi G, Landi A, Natuzzi F, Siri C, et al. A 5-year prospective assessment of advanced Parkinson disease patients treated with subcutaneous apomorphine infusion or deep brain stimulation. J Neurol. 2011;258(4):579–85.
Fabbri M, Rosa MM, Ferreira JJ. Adjunctive therapies in Parkinson’s disease: How to choose the best treatment strategy approach. Drugs Aging. 2018;35(12):1041–54.
Nyholm D, Odin P, Johansson A, Chatamra K, Locke C, Dutta S, et al. Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J. 2013;15(2):316–23.
Nyholm D, Askmark H, Gomes-Trolin C, Knutson T, Lennernas H, Nystrom C, et al. Optimizing levodopa pharmacokinetics: intestinal infusion versus oral sustained-release tablets. Clin Neuropharmacol. 2003;26(3):156–63.
Fasano A, Gurevich T, Jech R, Kovacs N, Svenningsson P, Szasz J, et al. Concomitant medication usage with levodopa-carbidopa intestinal gel: results from the COSMOS Study. Mov Disord. 2021;36(8):1853–62.
Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2012;367(16):1529–38.
OECD (2021). Purchasing Power Parities (PPP) (indicator). https://doi.org/10.1787/1290ee5a-en. https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm.
Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VS, Klostermann F, et al. Levodopa-carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord. 2015;30(4):500–9.
Fernandez HH, Boyd JT, Fung VSC, Lew MF, Rodriguez RL, Slevin JT, et al. Long-term safety and efficacy of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease. Mov Disord. 2018;33(6):928–36.
Palhagen SE, Sydow O, Johansson A, Nyholm D, Holmberg B, Widner H, et al. Levodopa-carbidopa intestinal gel (LCIG) treatment in routine care of patients with advanced Parkinson’s disease: an open-label prospective observational study of effectiveness, tolerability and healthcare costs. Parkinsonism Relat Disord. 2016;29:17–23.
Meira B, Degos B, Corsetti E, Doulazmi M, Berthelot E, Virbel-Fleischman C, et al. Long-term effect of apomorphine infusion in advanced Parkinson’s disease: a real-life study. NPJ Parkinsons Dis. 2021;7(1):50.
Dafsari HS, Martinez-Martin P, Rizos A, Trost M, Dos Santos Ghilardi MG, Reddy P, et al. EuroInf 2: subthalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord. 2019;34(3):353–65.
Soileau M, Pagan F, Fasano A, Rodriguez-Cruz R, Oh M, Kandukuri P, et al. Pill burden reduction in patients with advanced Parkinson's disease: comparative effectiveness of carbidopa/levodopa enteral suspension and deep brain stimulation. In: MDS virtual conference 2020;2020.
Boyd JT, Zadikoff C, Benesh JA, Zamudio J, Robieson WZ, Kukreja P. Saferty and efficacy of levodopa-carbidopa monotherapy in patients with advanced Parkinson's disease. In: XXIII World congress of neurology; September 16–21, 2017; Kyoto, Japan 2017.
Santos-Garcia D, de Deus FT, Suarez Castro E, Aneiros Diaz A, McAfee D. 5-2-1 criteria: a simple screening tool for identifying advanced PD patients who need an optimization of Parkinson’s treatment. Parkinsons Dis. 2020;2020:7537924.
Aldred J, Anca-Herschkovitsch M, Antonini A, Bajenaru O, Bergmann L, Bourgeois P, et al. Application of the “5-2-1” screening criteria in advanced Parkinson’s disease: interim analysis of DUOGLOBE. Neurodegener Dis Manag. 2020;10(5):309–23.
Rodríguez-Blázquez C, Forjaz MJ, Lizán L, Paz S, Martínez-Martín P. Estimating the direct and indirect costs associated with Parkinson’s disease. Expert Rev Pharmacoecon Outcomes Res. 2015;15(6):889–911.
Martinez-Martin P, Reddy P, Katzenschlager R, Antonini A, Todorova A, Odin P, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30(4):510–6.
Shalev V, Goldshtein I, Halpern Y, Chodick G. Association between persistence with statin therapy and reduction in low-density lipoprotein cholesterol level: analysis of real-life data from community settings. Pharmacotherapy. 2014;34(1):1–8.
Olivola E, Fasano A, Varanese S, Lena F, Santilli M, Femiano C, et al. Continuous subcutaneous apomorphine infusion in Parkinson’s disease: causes of discontinuation and subsequent treatment strategies. Neurol Sci. 2019;40(9):1917–23.
Moes HR, Groenendal-Laurensse J, Drent M, Tissingh G, van Laar T. Predictors of time to discontinuation of levodopa-carbidopa intestinal gel infusion: a retrospective cohort study. J Parkinsons Dis. 2020;10(3):935–44.
Constantin VA, Szasz JA, Orban-Kis K, Rosca EC, Popovici M, Cornea A, et al. Levodopa-carbidopa intestinal gel infusion therapy discontinuation: a ten-year retrospective analysis of 204 treated patients. Neuropsychiatr Dis Treat. 2020;16:1835–44.
Acknowledgements
The authors thank Ali Alobaidi, of AbbVie Inc., for his contributions to the manuscript.
Funding
This study was funded by AbbVie Inc., who also provided funding for the journal’s Rapid Service and Open Access Fees.
Medical Writing Assistance
Medical writing assistance, funded by AbbVie, was provided by James Street, PhD, Alicia Salinero, PhD, and Kersten Reich, MPH, CMPP™, of JB Ashtin.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Avner Thaler, Ruth Gross, Raanan Cohen, Lars Bergmann, Yash J Jalundhwala, Gabriel Chodick, and Tanya Gurevich contributed to study concept and design. Yael Barer was involved in data acquisition and statistical analysis. Avner Thaler, Ruth Gross, Raanan Cohen, Lars Bergmann, Yash J Jalundhwala, Nir Giladi, Varda Shalev, Tanya Gurevich were involved in data interpretation. All authors participated in writing, review, and critique of the manuscript. All authors had access to the data and participated in the development, review, critique, and approval of the manuscript throughout the editorial process, and approved the final manuscript draft submitted for publication. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication.
Prior Publication
Data from the study were previously presented at the 6th Congress of the European Academy of Neurology, 23–26 May 2020, Virtual.
Disclosures
Avner Thaler served as a consultant and/or scientific advisor for AbbVie and has received research support from the Michael J Fox Foundation. Yael Barer, Gabriel Chodick, and Varda Shalev report no conflict of interest. Ruth Gross is a former employee of AbbVie, currently employed by BOL Pharma LTD. and may hold AbbVie stock. Raanan Cohen, Lars Bergmann, and Yash J. Jalundhwala are employees of AbbVie and may hold stock and/or stock options. Nir Giladi serves as consultant to Sionara, NeuroDerm, Pharma2B, Denali, Neuron23, Sanofi-Genzyme, Biogen, and AbbVie. He receives royalties from Lysosomal Therapeutics (LTI) and payment for lectures at AbbVie, Sanofi-Genzyme, and the Movement Disorder Society. He has received research support from the Michael J Fox Foundation, the National Parkinson Foundation, the European Union, the Israel Science Foundation, Teva NNE program, Biogen, and Ionis. He receives support from the Sieratzki Family Foundation and the Aufzien Academic Center at Tel Aviv University. He reports no conflict of interest related to this work. Tanya Gurevich served as a consultant and/or scientific advisor for Neuroderm, AbbVie, Cytora, Synnerva, Allergan, Teva, and Medison. She has received research support from Parkinson’s Foundation, Israel Innovation Authority, and BrainBoost Program at the Sagol School of Neuroscience, Tel Aviv University.
Disclosures
AbbVie participated in the study design; study research; collection, analysis, and interpretation of data; and writing, reviewing, and approving this manuscript for submission. AbbVie funded the research for this study and provided writing support for this manuscript. No honoraria or payments were made for authorship. All authors had access to the data, were involved in manuscript development, and agreed to publish this manuscript.
Compliance with Ethics Guidelines
The study was approved by the Bayit balev internal review board committee of MHS (reference number 0057-18-BBL), and patient data were anonymized and de-identified before undergoing analysis. Bayit balev of the Maccabi Group is the committee of the MHS and approves Maccabi's studies, including the research use of Maccabi's databases.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and clinical study reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and statistical analysis plan and execution of a data sharing agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Thaler, A., Barer, Y., Gross, R. et al. Long-Term Persistence and Monotherapy with Device-Aided Therapies: A Retrospective Analysis of an Israeli Cohort of Patients with Advanced Parkinson’s Disease. Adv Ther 39, 2009–2024 (2022). https://doi.org/10.1007/s12325-022-02072-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02072-x