Abstract
Introduction
The rate of awareness of prospective prescription review for inpatient prescriptions remains low, and no study has evaluated prospective prescription review systems among hospitalized patients. In this study we evaluate the effect of a prospective prescription review system on the use of analgesics, clinical outcomes, and medical costs in hospitalized patients who underwent surgery.
Methods
A single-center, real-world study was conducted retrospectively at Drum Tower Hospital, Nanjing, China. Patient data were extracted from the medical records, before (June 2016–May 2017) and after (June 2018–May 2019) prescription review system implementation. The primary outcome was proportion of prescriptions of analgesics with potential risks. The secondary outcomes included prescription of opioids or non-opioids, usage of medications to manage analgesics-related adverse events, clinical outcomes, and medical costs. Propensity score matching was used to balance the cohort of patients before and after implementation of the prescription review system.
Results
A total of 28,150 inpatients were included for study analysis. After implementation of the prescription review system, the proportion of prescriptions of analgesics with potential risk was significantly reduced (6.3% vs 26.1%, P < 0.05). A significant decrease was observed in the proportion of patients prescribed opioids (24.3% vs 27.5%, P < 0.001) and tramadol (4.7% vs 12.1%, P < 0.001). There was a significant decrease in prescription of antiemetics (21.8% vs 34.1%, P < 0.001) and cathartics (38.4% vs 50.6%, P < 0.001) which were used in the management of opioid-related adverse events. There was a decreased length of stay in hospital [median (Q1, Q3) 10 (6, 17) vs 11 (7, 18), P < 0.01)] with similar readmission rates within 30 days post discharge (1.0% vs 0.8%, P = 0.099).
Conclusions
The introduction of the prescription review system was associated with safer prescribing, including a reduction in prescriptions of analgesics with potential risk and necessity of medication to manage analgesics-related adverse events, which resulted in better clinical outcomes and cost saving.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The rate of awareness of prospective prescription review for inpatient prescriptions remains low, and no study has evaluated prospective prescription review systems among hospitalized patients. |
This study evaluated the effect of a prospective prescription review system on the use of analgesics, clinical outcomes, and medical costs in hospitalized patients who underwent surgery. |
In this single-center, real-world, retrospective study of medical records covering 28,150 inpatients who underwent surgeries found that after prescription review system implementation, the proportion of prescriptions with potential risk of analgesics was significantly reduced. |
The proportion of patients prescribed opioids and tramadol significantly decreased while the proportion of patients prescribed acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) increased. |
The length of stay in hospital, readmission rates within 30 days post discharge, and cost of medication and medical devices related to pain management were also reduced. |
Introduction
According to China’s National Bureau of Statistics, more than 69 million hospitalized patients underwent surgery in 2019, of which a large population required pain management [1]. Optimal pain management allowing early mobilization is a prerequisite for faster postoperative recovery and lesser postoperative complications, which shortens length of stay at hospital and reduces cost of care [2]. However, use of analgesics with potential risk is becoming a critical issue in clinical practice. Addressing and solving this issue is necessary, not only to improve the provision of healthcare to ensure patient safety but also to allow optimal resource utilization [3]. Though analgesics are safe to use and the risks among these patients could be predictable, an important number of preventable adverse drug reactions due to analgesics are still detected [4]. The commonly observed adverse events due to overconsumption of nonsteroidal anti-inflammatory drugs (NSAIDs) include gastrointestinal (GI) ulcers or bleeds. These risk factors are usually dependent on drug dosage and duration of treatment, previous history of GI disorder as well as possibly concomitant Heliobacter pylori infection [5]. Opioid abuse is a prominent manifestation of the use of analgesics with potential risk in the USA [6]. The USA has the highest consumption of the opioids worldwide [7] and prescribes over 50 times more opioids, which has resulted in addiction and death at an epidemic level [8]. With regards to opioid consumption, the average morphine equivalent (MEs) in China was 2.86 mg per capita in 2010 compared to 782 mg per capita in the USA [9]. The regulations on the administration of anesthetic drugs and psychotropic drugs were issued by the Chinese government in 2005 to ensure legitimate medical use, and prevent illegal abuse of opioids. Moreover, the National Health and Family Planning Commission (NHFPC) of China launched the Good Pain Management (GPM) program in 2011[10]. Despite these regulations, there is a scarcity of studies on analgesics consumption in China and some hospitals have reported prescriptions of opioids (e.g., dezocine) [11] and non-opioids (e.g., NSAIDs) that carry enhanced risks of adverse events [12]. A multicenter retrospective study initiated by pharmacists in 2009 investigated the utilization of analgesics in 51 general hospitals nationwide, and found a large proportion of patients with overdose of patient-controlled analgesic (PCA) pump containing a variety of formulations, NSAIDs mixed with antiemetics and opioids [13].
Utilization of analgesics may lead to potential risks, increase the incidence of analgesics-related adverse events, impair patients’ recovery, and increase medical cost [14]. There are numerous reasons that can lead one to prescribe an analgesic that may pose a risk to the patient. As a result of the availability of various analgesics, including in different dosage forms (such as oral, injection, topical, and other forms) and different formulas (single preparation, compound preparation), duplication of doses or overdose can easily occur. On the other hand, surgical patients often suffer from comorbid diseases such as hypertension and diabetes, and during the perioperative period often require a combination of anticoagulants, antibiotics, and other drugs, which often generate adverse drug–drug interactions or increase the risk of adverse drug events. Given the reality of complicated perioperative medication, clinical pharmacists, a newly introduced role of pharmacist in China, who master the professional knowledge of drug utilization and participate in the therapeutic management of clinical patients, can play a professional role in promoting the safer prescription of drugs (including analgesics).
Hitherto, two important ways have been devised to improve efficient drug prescription patterns, mainly prescription comment and prescription review. A prescription review system usually identifies prescriptions with potential analgesic risk which includes high analgesic dose, co-prescribing of analgesic with other medications (or exceeding a specified duration of concurrent use), overlapping analgesic prescriptions, use of long-acting prescription analgesic formulations for acute pain, and early prescription refills. It aids in continuous improvement of the quality of medical care and the management of clinical application of drugs in hospitals. In 2010, the Ministry of Health initiated the “Management Standards for Hospital Prescription Comment (Trial)” [15] to standardize the process of hospital prescription comment. Prescription comment is a comprehensive retrospective evaluation on the standardization of prescriptions, drug indications, and contraindications, drug selection, route of administration, usage and dosage, and drug interactions, in accordance with relevant regulations and technical specifications for standardized prescription writing and the rational use of drugs. As a standard practice, the hospital prescription evaluation team randomly reviews prescriptions according to a prescribed sampling method, and makes comments on emergency prescriptions [15]. However, as prescription comment is a post-event, it cannot provide real-time feedback or intervene before drug administration. Furthermore, it depends on manual evaluation by pharmacists which consumes a lot of time; thus, prescription comment can only be assessed in a limited sample. The National Health Commission and two other departments jointly formulated the “Prescription Review Specification for Medical Institutions” [15] on June 29, 2018 which recommended that all prescriptions should be reviewed and approved by pharmacists before the dispensing process. Considering the massive number of prescriptions issued in outpatient and inpatient settings, a faster and more efficient approach is indispensable to conduct the prescription review.
To meet the demand for real-time prescription review, a prospective prescription review system is currently used to assist pharmacists. Pharmacists set the prescription review rules which are embedded into the hospital information system. The review rules mainly refer to drug specifications, laws and regulations, clinical treatment guidelines, clinical pathways, national prescription set, etc. When physicians submit electronic prescriptions, the review system conducts real-time and rapid prescription review to intercept the prescriptions with potential risks according to the review rules. If the prescription passes all the review rules, it will enter the dispensing process. If it does not comply, e.g., risk of overdose or contraindication, alerts will be immediately displayed and accordingly the physician must revise or cancel the prescription. For prescriptions which seem “uncertain” or are deemed of potential risk, e.g., potential drug–drug interaction, the prescriptions will be cleared only after pharmacists’ review. The pharmacist will review the “uncertain” prescriptions with potential risk, and either approve or reject and request revision, on the basis of their expertise of medication as well as the patients’ medical and treatment status (Fig. 1). In this manner, the pharmacists could intercept a prescription with potential risk before drug dispensing, and reduce the misuse of drugs.
Previously published studies showed that implementation of a prospective prescription review system significantly reduced the occurrence of prescriptions with potential risk in the outpatient setting, reduced the cost of prescription drugs, and improved patient satisfaction [16,17,18,19]. To date, most general hospitals in China have realized the importance of prospective prescription review for outpatient prescriptions; however, as a result of technical difficulties, the rate of awareness of prospective prescription review for inpatient prescriptions remains low. Moreover, no study has evaluated a prospective prescription review system among hospitalized patients.
Nanjing Drum Tower Hospital, one of the largest tertiary hospitals in eastern China, where more than 48,000 surgeries are performed per year (most of the surgeries come from spinal surgery and general surgery departments), launched a prescription review system (iPHARMACARE) in June 2018, which provides a real-time, comprehensive prescription review for the entire sample of outpatients and inpatients at the hospital.
Previous investigations have shown that patients in spinal surgery and general surgery departments may suffer from severe pain and need a large dose of analgesics. Thus, taking the samples from two wards of spinal surgery and general surgery departments before and after the implementation of the prospective prescription review system, this study analyzed the changes in prescription management, including the proportion of prescriptions of analgesics with potential risk, types of prescriptions with potential risk, use of concomitant medications to manage adverse events related to analgesics, as well as clinical outcomes and medical costs.
Methods
Study Design and Data Sources
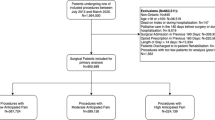
This single-center, real-world study was conducted retrospectively at Drum Tower Hospital, Nanjing, and approved by the Institutional Review Board of Nanjing Drum Tower Hospital (Approval number 2019-264-01). The study research period was designed according to the management time process of pharmacists in the hospital and was analyzed before and after prescription review system implementation. Data were collected from the medical records of patients over two time periods: before prescription review system implementation (from June 2016 to May 2017) and after system implementation (from June 2018 to May 2019). The medical data included information related to demographics, diagnosis, prescription patterns, laboratory examinations, duration of operation, and detailed medical expenses. On the basis of the objective of the study, prescriptions of the patients hospitalized for spinal surgery and general surgery were assessed for use of analgesics with potential risk and other clinical outcomes. Patients admitted to the departments of spinal surgery and general surgery of Drum Tower Hospital from June 2016 to May 2017 and June 2018 to May 2019 for inpatient surgery were selected at the initial step and then those who were prescribed analgesics after operation were finally included. Patients were excluded if they underwent one day surgery and minimally invasive surgery or if the patients had an admission time less than 48 h.
Study Variables
Analgesic prescribing with potential risk was defined as inadequate, continued, or excessive prescribing and that potentially poses high risks of morbidity and mortality [20]. These prescriptions were considered of potential risk which included either high analgesic dose, co-prescribing of analgesic with other medications, overlapping analgesic prescriptions, use of long-acting prescription analgesic formulations for acute pain, and early prescription refills. The primary outcome variable evaluated was the proportion of prescriptions of analgesics with potential risk. The secondary outcome variables were types of prescriptions with potential risk, use of concomitant medications to manage adverse events related to analgesics, clinical outcomes (length of stay at hospital, readmission rates within 30 days of discharge), and medical costs (medication and medical devices, such as PCA pump, related to pain management).
The outcome variables were defined as follows:
Proportion of prescriptions of analgesics with potential risk: the proportion of analgesia prescriptions with potential risk in patients’ perioperative analgesia prescriptions was reprogrammed and run on the basis of the latest version of review rules (the prescriptions were identified from the prescription data in the health information system (HIS), and the prescription review rules were provided by Drum Tower Hospital).
Types of prescriptions with potential risk: prescription review rules according to the Drum Tower Hospital, to classify the reasons for prescriptions with potential risk, including the improper selection of drugs, incorrect or excessive drug dosage, improper combination of analgesics with other concomitant drugs, incorrect route of drug administration (prescription recognition from the prescription data in HIS, prescription review rules provided by the Drum Tower Hospital).
Use of concomitant medications to manage adverse events related to analgesics: information related to opioid-related events: antiemetics and cathartics; NSAIDs-related events; gastric antisecretory agents.
Length of stay at hospital: discharge time–admission time in days, discharge time and admission time are identified from HIS visit data.
Readmission rates within 30 days of discharge: proportion of all-cause orthopedic/general surgical readmissions within 30 days of the first hospitalization (identified from HIS visit data).
Medical costs (medication and medical devices, such as PCA pump, related to pain management): Patients with analgesic drug costs (i.e., when the prescription of analgesic drugs during hospitalization expenses, excluding PCA pump cost, cost of recognition from HIS data) and prevention drug costs of opioid/ NSAIDs-related adverse events (i.e., the time when the patient is in the hospital and prescribed antiemetics, cathartics, gastric antisecretory agents, identified from the HIS data).
Drug dose: total dose of each analgesic drug used at the time of hospitalization (identified from prescription data in HIS).
For the drug dose calculation, the doses of different opioids were converted to oral morphine equivalent (OME), while for the dose of acetaminophen, the dose of propacetamol was converted into acetaminophen (conversion factor: propacetamol 1 g = acetaminophen 0.5 g).
Statistical Analysis
In this study, propensity score matching (PSM) was used to adjust for significant differences in patient characteristics and reduce the influence of possible confounding factors. For the method of PSM, a logistic regression model was calculated with the covariate variables (age, gender, smoking/drinking history, BMI, comorbidities, concomitant medication, type of surgery, ward, and type of medical insurance) to obtain the scores. After estimating propensity scores, 1:1 nearest-neighbor matching without replacement was performed with a caliper of width equal to 0.02. The difference of potential confounding variables with P value of less than 0.05 was considered to be statistically significant for each test before and after PSM.
For other clinical outcomes, quantitative data were analyzed using number of patients (n), mean, standard deviation (SD), minimum (min), maximum (max), median (MED), upper quartile (Q1), and lower quartile (Q3). Continuous variables included age and were compared using independent sample t test as data was normally distributed and expressed as mean ± standard deviation (SD). The number of days in hospital had a skewed distribution and hence was expressed as median (IQR). The categorical variables were summarized using frequency and percentage. When 20% of the lattice frequency was less than 5, Fisher’s exact test was used, and chi-square test was used for determination of remaining parameters. Cochran–Mantel–Haenszel’s chi-square test and logistic regression analysis were used to analyze the influence of prescription management and other factors.
Results
Demographic and Baseline Characteristics
A total of 28,150 inpatients were included for the study analysis out of which 12,787 patients were enrolled before prescription review system implementation and 15,363 patients after prescription review system. The mean age of the overall population was 51.27 ± 18.25 years with 49.2% of male patients. About 44.3% of the inpatients had spinal surgery while 55.7% of the patients had undergone general surgery (Table 1). After adjustment for the demographic variables by PSM analysis, the baseline characteristics before and after implementation of the prescription review system were found to be comparable. There were no significant differences in age, gender, history of disease complications, site of surgery, and surgical department between before and after prescription review system implementation.
Incidence of Prescriptions of Analgesics with Potential Risk
The proportion of prescription of analgesics with potential risk after prescription review system implementation was significantly reduced (6.3% vs 26.1%, P < 0.05) (Table 2). The most common use of analgesic prescriptions with potential risk before the prescription review system were found to be flurbiprofen axetil prescriptions (n = 8612, 22.0%), which was significantly decreased (n = 1251, 3.08%; P < 0.001) after the implementation of the prescription review system.
Incorrect or excessive dosage (N = 7899, 20.2%) and improper combination of analgesics (N = 2235, 5.7%) were identified as the two most common types of prescriptions with potential risk of analgesics before the prescription review system implementation. The prescriptions of incorrect or excessive dosage of analgesics were intercepted after the implementation of the prospective prescription review system, with a significant decrease in the proportion of incorrect or excessive dosage (N = 221, 0.5%). Improper combination of analgesics (N = 2283, 5.6%) was identified as the most common type of analgesic prescription with potential risk after the system implementation. Improper combination of analgesics was defined as when any two analgesics which should not be prescribed in combination were prescribed on the same day. Here we observed that several prescriptions for sequential treatment were identified as “uncertain”. These prescriptions were approved after pharmacists’ review, whereas “confirmed” improper combination of analgesics, such as prescribing two kinds of NSAIDs at the same time, were rejected.
Trends in Analgesic Prescriptions Among Inpatients
A significant decreasing trend was observed in the proportion of patients prescribed opioids (24.3% vs 27.5%, P < 0. 001) and tramadol (4.7% vs 12.1%, P < 0.001) (Table 3) after the prescription review system implementation. After conversion to OME, the mean dose of opioids (326.88 ± 781.18 mg vs 246.35 ± 2618.22 mg, P = 0.085) increased, though this difference was not statistically significant (Supplementary Table 1), while the mean dose of tramadol significantly decreased (294.03 ± 501.31 mg vs 503.57 ± 502.65 mg, P < 0.001). Meanwhile, the proportion of patients with non-opioids, i.e., acetaminophen (10.9% vs 9.5%, P < 0.001) and NSAIDs (88.9% vs 87.1%, P < 0.001) prescriptions increased significantly. There was a decrease in the mean dose of acetaminophen (7790.75 ± 8306.08 mg vs 8363.28 ± 6453.1 mg, P = 0.05) after system implementation. The mean dose of two common NSAIDs, flurbiprofen axetil (605.29 ± 541.02 mg vs 900.86 ± 796.55 mg, P < 0.001) and parecoxib (261.9 ± 277.73 mg vs 271.08 ± 248.06 mg, P = 0.093), also decreased; however, the difference in parecoxib was not statistically significant.
Use of Concomitant Medications to Manage Adverse Events Related to Analgesics
There was a significant decrease in prescription of antiemetics (21.8% vs 34.1%, P < 0.001) and cathartics (38.4% vs 50.6%, P < 0.001) which were used in the management (prophylaxis and/or treatment) of opioid-related adverse events such as nausea, vomiting, and constipation (Table 2). Furthermore, it was observed that durations of treatment with antiemetics (2.17 ± 2.25 days vs 2.78 ± 3.01 days, P < 0.001) and cathartics (1.78 ± 1.73 days vs 2.20 ± 2.42 days, P < 0.001) were significantly reduced after prescription review system implementation (Supplementary Table 2).
Meanwhile, there was a significant increase in prescription of gastric antisecretory agents (73.3% vs 59.4%, P < 0.001) after prescription review system implementation. These gastric antisecretory agents mainly were prescribed on the same day of NSAIDs prescriptions (97.3% vs 88.7%, P < 0.001). However, the treatment durations of gastric antisecretory agents showed no significant difference (5.13 ± 5.23 days vs 5.01 ± 5.05 days, P = 0.18).
Changes in Clinical Outcomes
The length of stay in hospital was shortened significantly [median (Q1, Q3) 10 (6, 17) days vs 11 (7,18) days, P < 0.01)], with no significant difference in all-cause readmission rates among inpatients within 30 days after discharge (1.0% vs 0.8%, P = 0.099) after prescription review system implementation (Table 4).
Costs Related to Pain Management
The mean cost of commonly prescribed analgesics showed a significant reduction after prescription review system implementation as follows: opioids (mean ± SD, ¥ 415.67 ± 735.63 vs ¥ 512.26 ± 932.23, P < 0.01), NSAIDs (¥ 731.46 ± 686.68 vs ¥ 970.83 ± 875.87, P < 0.01), acetaminophen (¥ 422.62 ± 567.45 vs ¥ 745.92 ± 637.76, P < 0.01), and tramadol (¥ 18.7 ± 21.37 vs ¥ 28.59 ± 21.55, P < 0.01) (Supplementary Table 3).
An additional analysis was done to evaluate the cost associated with the management of analgesic-related adverse events. The mean healthcare expenditure reduced in the case of antiemetics (mean ± SD, ¥ 62.68 ± 56.94 vs ¥ 229.22 ± 287.35, P < 0.01) and gastric antisecretory agents (¥ 433.13 ± 683.75 vs ¥ 724.52 ± 863.84, P < 0.01) after prescription review system implementation while expenditure related to consumption of catharsis (¥ 37.9 ± 53.31 vs ¥ 17.14 ± 59.78, P < 0.001) significantly increased (Supplementary Table 4).
Discussion
Lack of adequate assessment and inadequate treatment remain the major factors affecting undertreatment of pain. There is ample evidence that the proper analgesics use, i.e., taking the right drug(s) at the right intervals, can provide effective pain relief for the majority of patients [21]. Usually, formal medication reviews and deprescribing are considered time and resource intensive, and they are undertaken infrequently in clinical practice for several reasons. There may be underappreciation of the risks of polypharmacy, clinical inertia, limited pharmacology knowledge or self-confidence in deprescribing, or limited time and information about the particulars of individual patients. Prescribing drugs involves weighing up the benefits and risks; careful decision-making is needed to improve their safe use. The potential for these barriers can be overcome by an online prospective prescription review system aimed at reducing adverse events and improving patient safety.
In this study, we evaluated the effectiveness of a prospective prescription review system on the use of analgesics, clinical outcomes, and medical costs in hospitalized patients undergoing surgery. A national drug trend utilization analysis in China at 793 hospitals from 2013 to 2018 reported that the annual clinical drug dosage of NSAIDs increased by about 0.6 times whereas it was more than double for opioids [22]. Therefore, it is imperative to develop a rational guideline for safe use of analgesics. Moreover, a cross-sectional study in Australia revealed that after implementation of a policy to reduce prescriptions with potential risk, the overall dispensing of alprazolam decreased by 51.2% and prescribing approvals increased by 17.5% [23]. Similarly, following implementation of the prospective prescription review system at Drum Tower Hospital, China, the use of analgesic prescriptions with potential risk reduced from 26.1% to 6.3%, with a significant decrease in the proportion of prescriptions for opioid analgesics, accompanied by a moderate increase in non-opioids. Besides, the mean dose of opioid slightly increased. There was a decreasing trend in the proportion of antiemetics and cathartics which were used in the management of opioid-related adverse events. These findings were consistent with other published studies which reported that establishment of a drug utilization review (DUR) reduced the incidence rate of prescriptions with potential risk [24,25,26,27].
NSAIDs are considered to be the most commonly prescribed drugs for the management [28] of pain and inflammation, and this was also reflected in our study. However, NSAIDs are associated with gastrointestinal toxicity that limits their clinical use. In our study, despite the implementation of the prescription review system, there was an increase in the use of NSAIDs as well as gastro antisecretory agents. Furthermore, most gastric antisecretory agent prescriptions were prescribed on the same day as NSAIDs prescriptions, suggesting that surgeons may have paid more attention to the adverse drug events caused by NSAIDs and took early prophylaxis after prescription review system implementation. Several factors have contributed to opioid overprescribing including emphasis on chronic pain, and ties to reimbursement resulting in repeated use and addiction among patients [8]. In our study, we observed that there was a decrease in the prescription of opioid-related analgesics post adoption of the prescription review system. These findings were similar to a systematic literature review which addressed a positive association in reduction of opioid-related adverse events owing to prescription drug monitoring programs [29]. Another study reported a 24% change in more efficient prescribing of therapeutic agents with the DUR system [26]. Thus, reviewing of prescription analgesics in real time can reduce the prescription of drugs with potential risk and improve the quality of life of the patient.
Usually, flurbiprofen axetil is associated with a decrease in renal function. Recently, a retrospective data analysis of 9915 cases reported that there is a dose-dependent effect of flurbiprofen axetil on postoperative acute kidney injury and low dose may effectively reduce the impact [30]. In line with these findings, in our study the flurbiprofen axetil prescriptions were significantly decreased from 22.0% to 7.3% after the implementation of the prescription review system which may be attributed to renal-related side effects postoperatively.
Studies have shown that pharmacist-led prescription review leads to reduced hospitalization stay [31] and decreased readmission rates from 14.64% to 12.58% [32]. These findings were similar to our study where there was a significant shorter duration of hospitalization without significant increase in readmission rates. A survey among physicians and pharmacists on real-time monitoring of drug utilization reported 71% satisfaction with the use of this system. However, physicians often tend to ignore the important alerts leading to change in prescriptions and medications-related errors [33]. This necessitates an increased awareness and stringent guidelines for monitoring of drug prescriptions in real time as it captures variations in patterns of prescribing and consumption of drugs, reduces concern about delayed adverse effects along with cost of drugs and volume of prescription [34].
Limitations
To the best of our knowledge, our study is the first to evaluate the effect of a prescription review system on incidence of analgesic prescriptions with potential risk, clinical outcomes, and healthcare expenditure. However, there are some limitations in the study as well with the use of the prospective prescription review system. Firstly, the system can only review the prescriptions prescribed by surgeons in the wards (it is unrealistic to review the prescriptions during operations); thus, this study can only reflect in part of the whole picture of how analgesics are used in the real world. Secondly, this study evaluated the effect of the prospective prescription review system on the safe use of analgesics, clinical outcomes, and medical costs. However, as a result of a lack of electronic data on pain score, the effect of the prescription review system on the postoperative analgesic effect could not be evaluated. Thirdly, the decision to use analgesic prescriptions with potential risk depends on many factors such as patient history of disease, previous and current medication, laboratory and imaging examinations; thus, it is difficult to judge accurately the effective use of prescription only through the prospective prescription review system. Moreover, with the invention of new drugs and new understanding of old drugs, the rules in the system need to be inspected regularly and upgraded in a timely manner. Therefore, it is necessary to carry out prescription review in combination with comprehensive prescription comment by pharmacists, to identify potential problems and provide suggestions for continuing quality improvement of appropriate drug use.
Conclusion
The introduction of the prescription review system was associated with safer prescribing, including a reduction in prescriptions of analgesics with potential risk and necessity of concomitant medication to manage analgesics-related adverse events, which resulted in better clinical outcomes and cost saving. Further studies are needed to evaluate the long-term effects of the prescription review system in China.
References
Annual Data. https://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0O0A&sj=2018. Accessed Dec 18, 2020.
Ramsay MAE. Acute postoperative pain management. Proc (Bayl Univ Med Cent). 2000;13:244–7.
Ofori-Asenso R, Agyeman AA. Irrational use of medicines—a summary of key concepts. Pharmacy (Basel). 2016;4(4):35. https://doi.org/10.3390/pharmacy4040035.
Bénard-Laribière A, Miremont-Salamé G, Pérault-Pochat M-C, Noize P, Haramburu F, EMIR Study Group on behalf of the French Network of Pharmacovigilance Centres. Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Fundam Clin Pharmacol. 2015;29:106–11.
Moore N, Charlesworth A, Van Ganse E, et al. Risk factors for adverse events in analgesic drug users: results from the PAIN study. Pharmacoepidemiol Drug Saf. 2003;12:601–10.
U.S. Department of Health and Human Services. What is the U.S. opioid epidemic?. HHS.gov. 2017. https://www.hhs.gov/opioids/about-the-epidemic/index.html. Accessed Dec 18, 2020.
Suda KJ, Durkin MJ, Calip GS, et al. Comparison of opioid prescribing by dentists in the United States and England. JAMA Netw Open. 2019;2:e194303–e194303.
Theisen K, Jacobs B, Macleod L, Davies B. The United States opioid epidemic: a review of the surgeon’s contribution to it and health policy initiatives. BJU Int. 2018;122:754–9.
Schuchat A, Houry D, Guy GP. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425–6.
Fang W, Liu T, Gu Z, Li Q, Luo C. Consumption trend and prescription pattern of opioid analgesics in China from 2006 to 2015. Eur J Hosp Pharm. 2019;26:140–5.
Xiaoxiao L, Qiang Z, Jingyan. Analysis of unreasonable application of dezocine in the orthopedics department of our hospital. Jiangxi Med J. 2018;53:1325–7.
Rong-Rong W, Yue-feng R, Zhen-wei Y. A multi-center retrospective study on the rational use of non-steroidal anti-inflammatory drugs in the perioperative period—China Journals Full-text Database. Chin Pharm J. 2019;54:1104–8.
Bo Z, Jinyan, Hong G, et al. Analysis of postoperative analgesic use in adult inpatients in 51 comprehensive tertiary hospitals. Chin Pharm J. 2010;1959–62. https://global.cnki.net/kcms/detail/detail.aspx?filename=ZGYX201024022&dbcode=CJFQ&dbname=CJFD2010&v=.
Builders M. Rational and irrational use of analgesics: A review. 2016;4(7):55–60.
Notice of the Ministry of Health on Printing and Distributing the “Management Standards for Hospital Prescription Reviews (Trial)”. http://www.nhc.gov.cn/yzygj/s3590/201810/6103f922f61440d1b48ba1571b6b6b72.shtml. Accessed Oct 30, 2020.
Xin L, Lina L, Yanhong C, Xia T, Wansheng C, Rong W. Application of pre-prescription review system in outpatient prescription review. Pract Pharm Clin Remedies. 2018;4:475–9. https://doi.org/10.14053/j.cnki.ppcr.201804028.
Zhang D, Zhao M, Yang M, Su Y, Shi H, Kang Y. Pre-intervention work practice of outpatient prescription based on artificial intelligence. J Clin Medicat. 2017;15(12):45–8. https://doi.org/10.3969/j.issn.1672-3384.2017.12.011.
Hua X. Information-based monitoring platform for rational use of antibiotics at a Chinese university hospital. Eur J Hosp Pharm Sci Pract. 2016;23:257–65.
Ji Z, Song W, Ai C. Practice and evaluation of pre-prescription review model. J Hosp Pharm. 2018;38(16):1743–6. https://doi.org/10.13286/j.cnki.chinhosppharmacyj.2018.16.16.
Kim B, Nolan S, Beaulieu T, Shalansky S, Ti L. Inappropriate opioid prescribing practices: a narrative review. Am J Health Syst Pharm. 2019;76:1231–7.
Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, editor. Patient safety and quality: an evidence-based handbook for nurses. Rockville: Agency for Healthcare Research and Quality (US); 2008. http://www.ncbi.nlm.nih.gov/books/NBK2658/. Accessed Sep 8, 2021.
Shi H, Chen X, Liu X, et al. National drug utilization trend of analgesics in China: an analysis of procurement data at 793 public hospitals from 2013 to 2018. J Pharm Policy Pract. 2021;14:45.
Schaffer AL, Buckley NA, Cairns R, Pearson S. Comparison of prescribing patterns before and after implementation of a national policy to reduce inappropriate alprazolam prescribing in Australia. JAMA Netw Open. 2019;2:e1911590–e1911590.
Monane M, Matthias DM, Nagle BA, Kelly MA. Improving prescribing patterns for the elderly through an online drug utilization review intervention: a system linking the physician, pharmacist, and computer. JAMA. 1998;280:1249–52.
Daubresse M, Gleason PP, Peng Y, Shah ND, Ritter ST, Alexander GC. Impact of a drug utilization review program on high-risk use of prescription controlled substances. Pharmacoepidemiol Drug Saf. 2014;23:419–27.
Kim SJ, Han K-T, Kang H-G, Park E-C. Toward safer prescribing: evaluation of a prospective drug utilization review system on inappropriate prescriptions, prescribing patterns, and adverse drug events and related health expenditure in South Korea. Public Health. 2018;163:128–36.
Starner CI, Norman SA, Reynolds RG, Gleason PP. Effect of a retrospective drug utilization review on potentially inappropriate prescribing in the elderly. Am J Geriatr Pharmacother. 2009;7:11–9.
Choudhury DK, Bezbaruah BK. Prescribing pattern of analgesics in orthopedic in-patient department at tertiary care hospital in Guwahati, Assam, Northeast India. Indian J Pharmacol. 2016;48:377–81.
Rhodes E, Wilson M, Robinson A, Hayden JA, Asbridge M. The effectiveness of prescription drug monitoring programs at reducing opioid-related harms and consequences: a systematic review. BMC Health Serv Res. 2019;19:784.
Wang D, Yang S-K, Zhao M-X, et al. Low dose of flurbiprofen axetil decrease the rate of acute kidney injury after operation: a retrospective clinical data analysis of 9915 cases. BMC Nephrol. 2020;21:52.
Hohl CM, Partovi N, Ghement I, et al. Impact of early in-hospital medication review by clinical pharmacists on health services utilization. PLoS ONE. 2017;12:e0170495.
Schoenbaum AE, Seckman C. Impact of a prescription drug monitoring program on health information exchange utilization, prescribing behaviors, and care coordination in an emergency department. Comput Inform Nurs. 2019;37:647–54.
Lee S-M, Lee S-O, Kim D-S. Physicians’ and pharmacists’ perceptions on real-time drug utilization review system: a nationwide survey. Int J Qual Health Care. 2017;29:634–41.
Jain S, Upadhyaya P, Goyal J, et al. A systematic review of prescription pattern monitoring studies and their effectiveness in promoting rational use of medicines. Perspect Clin Res. 2015;6:86–90.
Acknowledgements
Funding
This work was funded by Pfizer. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The journal’s Rapid Service Fee and Open Access Fee were funded by the authors.
Medical Writing and Editorial Assistance
The authors acknowledge Anwesha Mandal and Dr. Amit Bhat of Indegene Pvt Ltd, India for their medical writing and editorial assistance, which was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Dr Xie had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: Ge, Xie, Li, Geng. Critical revision of the manuscript for important intellectual content: Zhang, Peng. Statistical analysis: Xie, Li, Geng. Obtained funding: Ge. Study supervision: Ge.
Disclosures
Weihong Ge reported receiving research funding from Pfizer, none of the other authors (Han Xie, Haixia Zhang, Jie Peng, Li Li, Yuyu Geng) had any funding disclosures to be reported.
Compliance with Ethics Guidelines
The study was approved by the Institutional Review Board of Nanjing Drum Tower Hospital (Approval number 2019-264-01).
Data Availability
Additional data will be provided by the author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Xie, H., Zhang, H., Peng, J. et al. Prospective Prescription Review System Promotes Safe Use of Analgesics, Improves Clinical Outcomes, and Saves Medical Costs in Surgical Patients: Insights from Nanjing Drum Tower Hospital. Adv Ther 39, 441–454 (2022). https://doi.org/10.1007/s12325-021-01935-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01935-z