Abstract
Introduction
In patients with chronic obstructive pulmonary disease (COPD), treatment with long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) combination therapy significantly improves lung function versus LABA/inhaled corticosteroid (ICS). To investigate whether LAMA/LABA could provide better clinical outcomes than LABA/ICS, this non-interventional database study assessed the risk of COPD exacerbations, pneumonia, and escalation to triple therapy in patients with COPD initiating maintenance therapy with tiotropium/olodaterol versus any LABA/ICS combination.
Methods
Administrative healthcare claims and laboratory results data from the US HealthCore Integrated Research Databasesm were evaluated for patients with COPD initiating tiotropium/olodaterol versus LABA/ICS treatment (January 2013–March 2019). Patients were aged at least 40 years with a diagnosis of COPD (but not asthma) at cohort entry. A Cox proportional hazard regression model was used (as-treated analysis) to assess risk of COPD exacerbation, community-acquired pneumonia, and escalation to triple therapy, both individually and as a combined risk of any one of these events. Potential imbalance of confounding factors between cohorts was handled using fine stratification, reweighting, and trimming by exposure propensity score (high-dimensional); subgroup analyses were conducted on the basis of blood eosinophil levels and exacerbation history.
Results
The total population consisted of 61,985 patients (tiotropium/olodaterol n = 2684; LABA/ICS n = 59,301); after reweighting, the total was 42,953 patients (tiotropium/olodaterol n = 2600; LABA/ICS n = 40,353; mean age 65 years; female 54.5%). Patients treated with tiotropium/olodaterol versus LABA/ICS experienced a reduction in the risk of COPD exacerbations (adjusted hazard ratio 0.76 [95% confidence interval 0.68, 0.85]), pneumonia (0.74 [0.57, 0.97]), escalation to triple therapy (0.22 [0.19, 0.26]), and any one of these events (0.45 [0.41, 0.49]); the combined risk was similar irrespective of baseline eosinophils and exacerbation history.
Conclusions
In patients with COPD, tiotropium/olodaterol was associated with a lower risk of COPD exacerbations, pneumonia, and escalation to triple therapy versus LABA/ICS, both individually and in combination; the combined risk was reduced irrespective of baseline eosinophils or exacerbation history.
Trial Registration
ClinicalTrials.gov identifier, NCT04138758 (registered 23 October 2019).
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Although some patients with chronic obstructive pulmonary disease (COPD) may benefit from regimens that include inhaled corticosteroids (ICS), ICS-containing treatments are often overprescribed and can increase the risk of pneumonia. |
To investigate whether combination therapy with the long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) tiotropium/olodaterol could provide better clinical outcomes than LABA/ICS, this large non-interventional database study assessed the risk of COPD exacerbations, pneumonia, and escalation to triple therapy in patients with COPD who initiated maintenance therapy with either combination. |
What was learned from the study? |
Overall, tiotropium/olodaterol was associated with a lower risk of COPD exacerbations, pneumonia, and escalation to triple therapy versus LABA/ICS, both individually and as a combined risk of any one of these events occurring; the combined risk was reduced versus LABA/ICS irrespective of baseline eosinophil count or exacerbation history. |
These results support and expand on those from previous randomized controlled trials that report a lower risk of exacerbations in subsets of patients with COPD treated with LAMA/LABA versus LABA/ICS. |
Our findings highlight the important role of LAMA/LABA in the management of COPD and implicate it as a strong alternative to LABA/ICS to avoid ICS overuse and reduce exacerbations in patients with COPD. |
Plain Language Summary
There are several different inhaled medicines for people with chronic obstructive pulmonary disease (COPD), including long-acting muscarinic antagonists (LAMAs), long-acting β2-agonists (LABAs), and inhaled corticosteroids (ICS). These medicines can be prescribed on their own or together. The most suitable choice of medicine depends on the person. For example, some people may benefit from treatments that include ICS, such as those who also have asthma or high levels of eosinophils—a type of white blood cell that can indicate inflammation in the body. However, ICS can also increase the risk of pneumonia. In this study, we compared treatment with a LAMA/LABA combination (called tiotropium/olodaterol) against LABA/ICS. We looked at which was more effective, also taking into account the risk of side effects. Using a large US database, we looked at information from over 40,000 patients with COPD who started treatment with either tiotropium/olodaterol or LABA/ICS. Overall, tiotropium/olodaterol reduced the risk of experiencing a worsening of symptoms (known as an exacerbation) compared with LABA/ICS. Tiotropium/olodaterol was also associated with a lower risk of pneumonia and the need to increase (or “escalate”) treatment to triple therapy with LAMA/LABA/ICS versus LABA/ICS. Looking at exacerbations, pneumonia, and escalation to triple therapy together, the risk of any one of these events was also reduced. This was true for patients with low or high eosinophil levels and for those who had a low or high number of exacerbations before starting treatment. Overall, these results suggest that a LAMA/LABA combination like tiotropium/olodaterol is a strong alternative to LABA/ICS.
Digital Features
This article is published with digital features, including a graphical abstract, summary slide, and plain language summary, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13677457.
Introduction
Multiple therapies can be used to treat chronic obstructive pulmonary disease (COPD) as long-term therapy, including long-acting bronchodilators (long-acting muscarinic antagonists [LAMAs] and/or long-acting β2-agonists [LABAs]) and inhaled corticosteroids (ICS). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020 strategy report recommends a stepwise approach to pharmacologic treatment, starting with a LAMA or LABA for most patients with COPD, and escalating to dual bronchodilation as the next step [1]. However, many patients remain symptomatic with monotherapy [2,3,4]. Recent guidelines from the American Thoracic Society go further, strongly recommending dual LAMA/LABA therapy over LAMA or LABA monotherapy for patients with COPD with dyspnea or exercise intolerance [5].
The optimal choice of treatment for COPD may depend on several factors. For instance, some patients, such as those with high eosinophil levels, an increased exacerbation risk, or a history of asthma, may benefit from regimens that include ICS [1, 5, 6]. The GOLD 2020 strategy report recommends that initial therapy with LABA/ICS is limited to patients with eosinophil levels of at least 300 cells/µL who are at high risk of exacerbations and are symptomatic [1].
Despite these recommendations, ICS are sometimes overprescribed and may be appropriate only in a subset of users [7,8,9,10,11,12,13,14,15]. In particular, ICS overuse is common in the USA, as shown by a cross-sectional study in the US Veterans Affairs system in which a quarter of patients with COPD without an identifiable indication for ICS had filled two or more prescriptions for ICS in the past year [16]. Notably, ICS use is widely associated with an increased risk of pneumonia and related use of healthcare resources and cost [8, 17,18,19,20], making it particularly important that ICS are prescribed appropriately. For example, triple therapy with LAMA/LABA/ICS has been associated with an increased risk of pneumonia and higher costs compared with dual therapy with LAMA/LABA combinations [5, 21]. Hence, escalation to triple therapy should be reserved for a specific population of patients with COPD, namely symptomatic patients with a high risk of exacerbations, in whom the treatment benefits may outweigh the risks [5, 22, 23].
Given the increasing evidence around the need for more tailored use of ICS in people with COPD [1, 5, 6, 24], it is important to investigate if non-ICS-containing regimens can provide the same or better clinical outcomes without the added risk of side effects associated with ICS. Treatment with LAMA/LABA combinations, including tiotropium/olodaterol, has been shown to significantly improve lung function versus LABA/ICS combinations [3, 25,26,27]. This non-interventional database study therefore aimed to assess the risk of COPD exacerbations, pneumonia, and escalation to triple therapy, both individually and as a combined risk of any one of these events occurring, in patients with COPD who initiated maintenance therapy with tiotropium/olodaterol versus any LABA/ICS combination.
Methods
Data Sources
This study (NCT04138758) was conducted using data from the HealthCore Integrated Research Database (HIRD; January 2013–March 2019), a large administrative healthcare claims database maintained by HealthCore that has previously been used for the study of numerous diseases, including COPD [28,29,30,31,32]. The study was conducted using administrative claims in the form of a limited data set pursuant to data agreements between HealthCore and participating health plans in compliance with the US Health Insurance Portability and Accountability Act. The study did not require ethics committee approval, nor were subjects required to provide informed consent.
HIRD includes longitudinal medical and pharmacy claims data for health plan members residing in the USA. Member enrollment, medical care (professional and facility claims), outpatient prescription drug use, outpatient laboratory test result data, and healthcare utilization may be tracked for health plan members in the database dating back to January 2006, and with diagnoses recorded in International Classification of Diseases, version 10 (ICD-10) since October 2015. Laboratory data are available for those tests that have been performed using two large, national reference laboratories (Quest and LabCorp).
Study Population and Design
Patients in each cohort were required to have at least one prescription for a fixed-dose combination (FDC) inhaler of tiotropium/olodaterol or LABA/ICS between 1 January 2013 and 31 March 2019, with the first prescription defined as the index date. Patients were also required to have at least one diagnosis of COPD at any time prior to the index date and at least 1 year of continuous health plan eligibility prior to the index date. Patients were excluded for the following reasons: age less than 40 years at the index date; diagnosis of asthma in the year prior to the index date, lung cancer, interstitial lung disease, or lung transplant; or pre-index use of tiotropium/olodaterol, LABA/ICS, or LABA/LAMA/ICS in free or fixed form.
Study Subpopulations
All analyses were conducted for the total population, as well as in subgroups of patients at high or low risk of exacerbation based on (1) previous history of exacerbations in the year preceding cohort entry (low exacerbation history, 0 hospitalizations and 0–1 outpatient exacerbations; high exacerbation history, at least 1 hospitalization or at least 2 outpatient exacerbations); and (2) blood eosinophil count (baseline eosinophils less than 300 cells/µL; baseline eosinophils at least 300 cells/µL) as identified on the basis of the laboratory result value that was closest but prior to the index date (within 6 months).
Exposure Measures
Exposure measures were based on pharmacy dispensation of tiotropium/olodaterol and LABA/ICS over up to 1 year of follow-up. Exposure was based on the current use of tiotropium/olodaterol or LABA/ICS as defined by the dispensation date plus the day’s supply recorded at the time of dispensation.
A gap of 15 days was allowed between dispensations to allow for delays in obtaining medication refills and for continued use beyond the days supplied where medication has been missed because of imperfect adherence. The 15-day gap was increased in the sensitivity analyses to 30 and 60 days.
Patients were followed from the index date until the earliest of the following: first occurrence of a COPD exacerbation, community-acquired pneumonia, escalation to triple therapy, switch in treatment, discontinuation of COPD treatment, the end of the study period, the end of continuous health plan eligibility, or (for the main analyses) 1 year after cohort entry. The treatment segment ended at the earliest of the following events: (1) 15 days after the end of the observed day’s supply for the medication received on the index date without a subsequent dispensing of COPD medication (i.e., discontinuation); (2) initiation of triple therapy; or (3) any other change in use of study medication, including to a different combination therapy, from a fixed-form to a free-form combination therapy, or from combination therapy to monotherapy.
Study Outcomes
The primary outcome was to compare the risk of first COPD exacerbation after initiating treatment with tiotropium/olodaterol versus LABA/ICS. COPD exacerbation was defined as a COPD-related hospitalization (severe exacerbation) or an emergency department visit for COPD and/or prescription of an antibiotic for a respiratory condition the same day as an oral corticosteroid (moderate exacerbation). To increase the specificity of our definition of exacerbation, the prescription of either an antibiotic or oral corticosteroid alone was not included. Severe and moderate exacerbations were considered as a composite for the main analyses.
Secondary outcomes included the risk of hospitalization for community-acquired pneumonia, risk of pharmacy dispensation indicating escalation to triple therapy, and risk of any of the following events occurring: COPD exacerbation, hospitalization for pneumonia, or escalation to triple therapy. Pneumonia was defined as hospitalization with one of the following diagnostic codes: 481.x–486.x, 487.0, 507.x, 507.0, 507.1, 507.8, 510.0, 510.9, 511.0, 513.0, 514.x, 517.1, 519.8, 530.84 (as per International Classification of Diseases, version 9, Clinical Modification [ICD-9-CM]; used up to October 2015) and J10.0, J11.0, J12–J18, J22, J69, J85.0, J85.1, and J86 (ICD-10; used from October 2015 onwards) (pneumonia without hospitalization was not captured). These definitions from hospitalization data have been used in several previous studies in patients with COPD [33] and are detailed in Table S1 in the supplementary material. As a result of the nature of the ICD diagnostic codes (e.g., J10.0: “influenza due to other identified influenza virus with pneumonia” [ICD-10]), it was not possible to verify pneumonia as the primary driver of hospitalization. Escalation to triple therapy was defined as any combination of pharmacy dispensations resulting in the overlapping use of a LAMA, LABA, and ICS through any fixed or free combination for at least 1 day.
Statistical Analysis
Outcome rates are described using incidence rates (IRs) and associated 95% confidence intervals (CIs). Differences in the risk of a first COPD exacerbation, pneumonia, escalation to triple therapy, or any one of these events (COPD exacerbation, or pneumonia, or escalation to triple therapy) for tiotropium/olodaterol use relative to LABA/ICS use were assessed using hazard ratios (HRs) and associated 95% CIs. Cox proportional hazard models were performed as an as-treated analysis to derive HRs; a first treatment carry-on analysis was conducted as a sensitivity analysis. Confounding was controlled via fine stratification and reweighting of an exposure propensity score and by including potential confounding factors in the models for variables that remained imbalanced after reweighting. Data trimming was applied on the basis of the overlap of propensity score distributions [34].
Data were analyzed separately and for subgroups based on blood eosinophil levels (for those with available results) and exacerbation history using the same approach. Calculation of propensity scores and fine stratification and reweighting were repeated within the patient subgroups to create weighted populations.
Propensity scores including both pre-specified and high-dimensional, data-derived variables were calculated; scores were calculated on the basis of the following covariates: sex, age, calendar year of cohort entry, season of index date, US census region of residence, insurance type, medication history, exacerbation history and pre-specified chronic comorbidities, in addition to the most frequently occurring diagnoses, medications, and procedures observed. Adjustment for the propensity score used the fine stratification method to create a weighted pseudo-population [34]. Ten strata were defined on the basis of the distribution of the propensity score within tiotropium/olodaterol-exposed individuals for the region of overlap with the LABA/ICS group, and stratum-specific weights were applied to create balance between the exposure groups. The balance of patient characteristics between the treatment groups was described before and after propensity score application. The Cox proportional hazard model was further adjusted for patient characteristics found to be imbalanced after application of the propensity score, where imbalance was defined as standardized differences greater than 10%. Several sensitivity analyses and bias analyses were performed, as described in the supplementary methods.
Results
Study Cohort
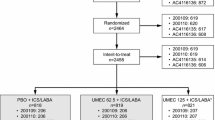
Data were available from 237,328 patients with a diagnosis of COPD who had received at least one dose of study medication from 1 January 2013 to 31 March 2019 (Fig. 1). After removing patients who did not meet the inclusion/exclusion criteria, there were 2684 tiotropium/olodaterol and 59,301 LABA/ICS users remaining. Of those, 361 tiotropium/olodaterol users (13.5%) and 6586 LABA/ICS users (11.1%) had at least one eosinophil test result recorded within 6 months prior to the index date. Based on pre- and post-index health plan eligibility data, the median time of enrollment was 61.1 months for tiotropium/olodaterol and 60.3 months for LABA/ICS. In the LABA/ICS group, the mean (± standard deviation) dose of ICS was 254.51 ± 242.31 µg and the majority of users (n = 56,558; 95.4%) received LABA/ICS via a single inhaler.
Formation of the study cohort. Percentage values show the proportion of individuals lost from the study cohort at each step compared with those with at least one COPD diagnosis (n = 237,328 [100%]). COPD chronic obstructive pulmonary disease, ICS inhaled corticosteroids, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, Olo olodaterol, Tio tiotropium
Baseline Characteristics
Baseline characteristics before and after reweighting are shown in Table 1. Prior to reweighting, the mean age of patients was 64.8 and 65.0 years for tiotropium/olodaterol and LABA/ICS, respectively; 45.6% of tiotropium/olodaterol users and 49.7% of LABA/ICS users were female. The majority of patients had not received previous maintenance treatments for COPD (tiotropium/olodaterol group, 69.2%; LABA/ICS group, 82.0%). The most common previous COPD treatment was LAMA monotherapy (tiotropium/olodaterol, 22.4%; LABA/ICS, 12.9%). More patients in the tiotropium/olodaterol group reported previous LAMA/LABA combination therapy versus those in the LABA/ICS group (6.4% versus 1.1%).
After reweighting for stratified propensity scores, the total pseudo-population consisted of 42,953 patients (tiotropium/olodaterol, 2600; LABA/ICS, 40,353). Overall, after reweighting, baseline characteristics were balanced between users of tiotropium/olodaterol and LABA/ICS, as demonstrated by low values of standardized difference (Table 1). There was still some imbalance in prior exacerbation history between tiotropium/olodaterol and LABA/ICS users, particularly for severe exacerbations (tiotropium/olodaterol versus LABA/ICS: one severe exacerbation in year prior to cohort entry, 13.2% vs. 18.3%; at least two exacerbations, 2.7% vs. 4.7%). To account for this imbalance, severe exacerbations were adjusted for in the model.
Risk of COPD Exacerbations
Tiotropium/olodaterol users had a lower adjusted IR (aIR [95% CIs]) per 100 person-years of exacerbations (59.53 [53.68, 66.03]) than LABA/ICS users (88.67 [86.45, 90.94]) (see Table 2 for adjusted and unadjusted data). The risk of exacerbations was also lower with tiotropium/olodaterol compared with LABA/ICS (adjusted HR [aHR] 0.76 [95% CI 0.68, 0.85]) (Fig. 2).
Risk of exacerbation, pneumonia, escalation to triple therapy, or a combination of these events (exacerbation, or pneumonia, or escalation to triple therapy) in patients receiving tiotropium/olodaterol versus LABA/ICS. Hazard ratios were derived using Cox proportional hazard models. The Cox proportional hazard model was further adjusted for patient characteristics found to be imbalanced after application of the propensity score, where imbalance was defined as standardized differences greater than 10%. aHR adjusted hazard ratio, CI confidence interval, COPD chronic obstructive pulmonary disease, ICS inhaled corticosteroids, LABA long-acting β2-agonist, Olo olodaterol, Tio tiotropium
Similar results were observed for patients stratified by baseline exacerbation frequency and for patients with baseline eosinophil levels less than 300 cells/µL (Table 2 and Fig. 3). Notably, the observation that tiotropium/olodaterol users had a lower risk of exacerbations than LABA/ICS users tended to be more pronounced in patients with baseline eosinophils less than 300 cells/µL (aHR 0.54 [95% CI 0.36, 0.82]) versus patients with at least 300 cells/µL (aHR 0.83 [0.50, 1.38]).
Subgroup analyses for the risk of exacerbations in patients receiving tiotropium/olodaterol versus LABA/ICS. Hazard ratios were derived using Cox proportional hazard models. The Cox proportional hazard model was further adjusted for patient characteristics found to be imbalanced after application of the propensity score, where imbalance was defined as standardized differences greater than 10%. aHR adjusted hazard ratio, CI confidence interval, ICS inhaled corticosteroids, LABA long-acting β2-agonist, Olo olodaterol, Tio tiotropium
Across sensitivity analyses, the aHR estimates varied from 0.69 to 0.91 (see Table S2 in the supplementary material). This was generally consistent across the subgroup analyses. For the bias analysis, only minimal changes in the aHR were observed when correcting for the parameters shown in Table S3 in the supplementary material.
Risk of Pneumonia
Tiotropium/olodaterol users had a lower aIR (95%CI) per 100 person-years of hospitalization for pneumonia (8.57 [6.61, 11.10]) than LABA/ICS users (12.54 [11.76, 13.36]) (see Table 2 for adjusted and unadjusted data). The risk of pneumonia was also lower with tiotropium/olodaterol compared with LABA/ICS (aHR 0.74 [95% CI 0.57, 0.97]) (Fig. 2).
Risk of Escalation to Triple Therapy
Tiotropium/olodaterol users had a lower aIR (95% CI) per 100 person-years of escalation to triple therapy (20.81 [17.53, 24.47]) than LABA/ICS users (114.79 [112.50, 117.25]) (see Table 2 for adjusted and unadjusted data). The risk of being escalated to triple therapy was also lower with tiotropium/olodaterol compared with LABA/ICS (aHR 0.22 [95% CI 0.19, 0.26]) (Fig. 2).
Combined Risk of COPD Exacerbation, or Pneumonia, or Escalation to Triple Therapy
When looking at the combined risk of any one of a COPD exacerbation, pneumonia, or escalation to triple therapy, tiotropium/olodaterol users had a lower aIR (95% CI) per 100 person-years (79.04 [72.32, 86.56]) than LABA/ICS users (207.21 [203.81, 210.75]) (see Table 2 for adjusted and unadjusted data). The risk of a COPD exacerbation, or pneumonia, or escalation to triple therapy was also lower with tiotropium/olodaterol compared with LABA/ICS (aHR 0.45 [95% CI 0.41, 0.49]; Fig. 2).
Similar results were observed for patients stratified by baseline exacerbation frequency and eosinophil levels (Table 2 and Fig. 4). For eosinophils, there was no notable difference between tiotropium/olodaterol and LABA/ICS in patients with fewer than 300 cells/µL (aHR 0.39 [95% CI 0.29, 0.53]) versus patients with at least 300 cells/µL (aHR 0.54 [0.34, 0.86]).
Subgroup analyses for the risk of exacerbation or pneumonia or escalation to triple therapy in patients receiving tiotropium/olodaterol versus LABA/ICS. Hazard ratios were derived using Cox proportional hazard models. The Cox proportional hazard model was further adjusted for patient characteristics found to be imbalanced after application of the propensity score, where imbalance was defined as standardized differences greater than 10%. aHR adjusted hazard ratio, CI confidence interval, ICS inhaled corticosteroids, LABA long-acting β2-agonist, Olo olodaterol, Tio tiotropium
Discussion
This non-interventional, real-world study showed that, in patients who initiated maintenance therapy with tiotropium/olodaterol versus LABA/ICS, there was a reduction in the risk of COPD exacerbations, community-acquired pneumonia and escalation to triple therapy, as well as a 54% reduction in the combined risk of any one of these events. The combined measure provides a useful and clinically relevant comparison of the two treatment options, given that the prescription of a treatment may be based on avoiding a number of possible events.
These findings are consistent with a growing body of evidence reporting that LAMA/LABA combination therapy is associated with a comparable or lower risk of exacerbations compared with LABA/ICS, while ICS use is linked with an increased risk of pneumonia [35,36,37,38]. For example, the FLAME trial reported that patients with COPD and a history of exacerbation receiving LAMA/LABA (indacaterol/glycopyrronium) had fewer exacerbations and a lower incidence of pneumonia than those receiving LABA/ICS (salmeterol/fluticasone) over 1 year of follow-up [35]. Similarly, in LANTERN, patients with moderate-to-severe COPD and a history of at least one exacerbation in the previous year who received indacaterol/glycopyrronium had a significantly lower rate of moderate-to-severe exacerbations and a threefold lower incidence of pneumonia compared with those receiving salmeterol/fluticasone [37]. In AFFIRM COPD, there was a comparable reduction in exacerbation risk, but a lower incidence of pneumonia, with LAMA/LABA (aclidinium/formoterol) versus salmeterol/fluticasone in patients with stable, moderate-to-severe COPD [38]. Additionally, several large observational studies have shown no benefit of LABA/ICS over LAMA or LAMA/LABA in terms of reducing exacerbation risk [39,40,41]. For example, in a large retrospective study in the USA evaluating 5384 patients on LAMA/LABA and 473,388 on LABA/ICS, both treatments were similarly effective in terms of exacerbation rates [39]. Similarly, in a recent real-world study comparing 1977 patients with COPD initiating LAMA/LABA with 1977 initiating LABA/ICS, LAMA/LABA was as effective as LABA/ICS in preventing exacerbations, but with a lower incidence of severe pneumonia [40]. Also, in a population-based observational study of 12,366 patients with COPD initiating LAMA and 12,366 initiating LABA/ICS, patients with low blood eosinophil levels (at most 300 cells/µL) experienced no difference in exacerbation risk with either treatment [41]. In contrast, in both the IMPACT and ETHOS trials, which compare triple LAMA/LABA/ICS therapy with LABA/ICS and LAMA/LABA, a lower rate of moderate or severe COPD exacerbations was observed with LABA/ICS versus LAMA/LABA [42, 43]. However, it is worth noting that these patients were more severe in terms of exacerbation history and lung function than those in the current study [42, 43]. Our results confirm that LAMA/LABA is the preferred option as initial therapy in patients with infrequent exacerbations.
There is also evidence to support the safe withdrawal of ICS where appropriate [24]. Recent guidelines from the European Respiratory Society (ERS) conditionally recommend withdrawing ICS in patients with COPD without a history of frequent exacerbations and strongly recommend treatment with one or two long-acting bronchodilators if ICS are withdrawn [24]. In support of this, in patients from the WISDOM trial with COPD and a history of exacerbations who received LAMA/LABA/ICS triple therapy (tiotropium/salmeterol/fluticasone propionate), the risk of moderate or severe exacerbations was similar among those discontinuing versus continuing ICS [36]. Notably, the ERS guidelines strongly recommend not withdrawing ICS in patients with blood eosinophil counts of 300 cells/µL or higher [24].
Given that LABA/ICS are currently recommended for symptomatic patients with higher exacerbation risk [1], lower exacerbation rates may be anticipated in LAMA/LABA versus LABA/ICS users, which has the potential to confound any comparison of these treatment regimens. However, the reduction in exacerbation risk with tiotropium/olodaterol versus LABA/ICS in our study occurred irrespective of baseline exacerbation history, indicating reductions in patients with both low and high exacerbation history. Similarly, prior therapy did not influence our results, as shown by a sensitivity analysis that included only those patients who had not received prior monotherapy; in this analysis, only marginal variations in the risk estimates for COPD exacerbations were observed overall and in the subgroup analyses by exacerbation history, suggesting that prior monotherapy did not modify the observations. Lastly, eosinophil count has the potential to influence exacerbation outcomes; however, our study showed that patients benefit from initiating therapy with tiotropium/olodaterol compared with LABA/ICS, irrespective of baseline eosinophil count. The reduction in COPD exacerbation risk with tiotropium/olodaterol versus LABA/ICS was more pronounced in patients with low baseline eosinophil levels (less than 300 cells/µL) compared with those with high baseline eosinophils (at least 300 cells/µL). Of note, a previous UK observational study reported no difference in exacerbation risk with LABA/ICS versus LAMA initiation in patients with COPD and low baseline eosinophil levels; however, a reduction in risk with LABA/ICS versus LAMA was observed in patients with high baseline eosinophils [41].
The increased risk of pneumonia with LABA/ICS versus tiotropium/olodaterol in our study is consistent with findings from a previous retrospective claims analysis, which showed that pneumonia risk was higher for ICS users versus non-users in Medicare patients with COPD in the USA [44]. An increased risk of pneumonia with LABA/ICS versus LAMA/LABA or LAMA was also observed in two previous real-world, clinical practice, observational studies [40, 41]. It is worth noting that the incidence rate of pneumonia was slightly higher in our study compared with previous studies [41, 45]; however, it was similar to that reported in a recent observational study by Suissa et al. [40]. Large variations in pneumonia rates in patients with COPD have been reported, for example between countries [46], which therefore makes it difficult to compare incidence rates between studies.
For escalation to triple therapy, our observation of a reduction in risk with tiotropium/olodaterol versus LABA/ICS is in line with results from a previous retrospective US health insurer database study showing that patients with COPD initiating LAMA/LABA (umeclidinium/vilanterol) had a longer time before escalation to multiple-inhaler triple therapy (and higher adherence) than those initiating fluticasone propionate/salmeterol [47].
A key strength of this study is the large number of patients (n > 40,000) in the main cohort and that only new initiators of tiotropium/olodaterol and LABA/ICS combinations were identified, allowing the study to reflect treatment outcomes relating to initial usage. Despite the substantial difference in the numbers of tiotropium/olodaterol and LABA/ICS users, cohorts were balanced after fine stratification, reweighting, and trimming by exposure propensity score, and large numbers of patients could be retained in the analysis. After reweighting, an imbalance in prior history of severe exacerbations remained between tiotropium/olodaterol and LABA/ICS users; however, this was accounted for by adjusting the model for severe exacerbations. To control for any confounding by treatment group, we used fine stratification and reweighting by exposure propensity score. However, this was only possible for measured covariates, and therefore an impact of residual confounding by unmeasured confounders cannot be ruled out. For instance, claims data are unlikely to capture any lifestyle factors that are less critical to insurance billing, as assessed in the bias analysis. In addition, eosinophil test results were only available in a subset of users, who may not be representative of the total study population. A further limitation is the lack of lung function data, which could have introduced unmeasured confounding by not allowing accurate reweighting by COPD disease severity. The model was, however, adjusted for severe exacerbations.
In addition, the definition used for moderate exacerbations did not include the prescription of either an antibiotic or an oral corticosteroid alone, and therefore under-reporting of COPD exacerbations was possible. However, it is more likely that only true exacerbation events were captured. A further limitation was that it was not possible to verify pneumonia as the primary driver of hospitalization because of the nature of the ICD diagnostic codes (e.g., J10.0: “influenza due to other identified influenza virus with pneumonia” [ICD-10]). The inclusion criteria present another potential limitation, since patients were included if they had at least one prescription of an FDC inhaler; those with only one prescription have an increased possibility of non-use or discontinuation when compared with multiple prescriptions. The prescriptions dispensed by a pharmacy but not taken by patients could lead to misclassification of exposure. Pharmacy dispensation data should more closely reflect patient use than physician prescribing given that the patient has taken the effort to obtain the medication. Quantitative bias analyses were used to formally describe the extent to which some of these issues are present. The analyses suggest that the impact of residual confounding due to unmeasured obesity or smoking status did not have a meaningful impact on study results. It is worth noting, however, that bias analysis parameters are informed by literature and clinical expert opinion; hence, any analysis is limited by such assumptions.
There is an inherent limitation in that the study population is limited to those with health insurance and hence the results may not be generalizable to uninsured patients with COPD. However, the sensitivity analyses that were conducted suggest that there was a relatively small variation in the risk estimates for COPD exacerbations overall and across the subgroup analyses. A further limitation is the possibility of information bias due to misclassification of outcomes, exposure, or missing data. In addition, for the subgroup analyses of COPD exacerbations and pneumonia, the relatively small sample sizes did not allow for strong conclusions to be drawn.
Conclusion
This analysis shows that treatment with tiotropium/olodaterol is associated with a lower risk of COPD exacerbations, pneumonia, and escalation to triple therapy, both individually and as a combined risk of any one of these events occurring, versus LABA/ICS in patients with COPD. Our findings support and expand on those from previous randomized controlled trials reporting a lower risk of exacerbations in subsets of patients with COPD treated with LAMA/LABA versus LABA/ICS.
These results highlight the important role of LAMA/LABA in the management of COPD, and implicate it as a strong alternative to LABA/ICS to avoid ICS overuse and reduce exacerbations in patients with COPD.
References
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report). 2019. https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf. Accessed 15 June 2020
Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904.
Miravitlles M, Urrutia G, Mathioudakis AG, Ancochea J. Efficacy and safety of tiotropium and olodaterol in COPD: a systematic review and meta-analysis. Respir Res. 2017;18(1):196.
Izquierdo JL, Miravitlles M, Esquinas C, et al. Characteristics of COPD patients managed in respiratory medicine departments in Spain, according to GOLD groups and GesEPOC clinical phenotypes. Arch Bronconeumol. 2018;54(11):559–67.
Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–69.
National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. 2019. https://www.nice.org.uk/guidance/ng115/chapter/Recommendation. Accessed 15 June 2020.
Yawn BP, Suissa S, Rossi A. Appropriate use of inhaled corticosteroids in COPD: the candidates for safe withdrawal. NPJ Prim Care Respir Med. 2016;26:16068.
Avdeev S, Aisanov Z, Arkhipov V, et al. Withdrawal of inhaled corticosteroids in COPD patients: rationale and algorithms. Int J Chron Obstruct Pulmon Dis. 2019;14:1267–80.
Suissa S, Rossi A. Weaning from inhaled corticosteroids in COPD: the evidence. Eur Respir J. 2015;46(5):1232–5.
Corrado A, Rossi A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med. 2012;106(7):989–97.
Roche N, Pribil C, Van Ganse E, et al. Real-life use of fluticasone propionate/salmeterol in patients with chronic obstructive pulmonary disease: a French observational study. BMC Pulm Med. 2014;14(1):56.
Drivenes E, Østrem A, Melbye H. Predictors of ICS/LABA prescribing in COPD patients: a study from general practice. BMC Fam Pract. 2014;15(1):42.
White P, Thornton H, Pinnock H, Georgopoulou S, Booth HP. Overtreatment of COPD with inhaled corticosteroids—implications for safety and costs: cross-sectional observational study. PLoS One. 2013;8(10):e75221.
Savran O, Godtfredsen N, Sorensen T, Jensen C, Ulrik CS. COPD patients prescribed inhaled corticosteroid in general practice: based on disease characteristics according to guidelines? Chron Respir Dis. 2019;16:1479973119867949.
Lucas AEM, Smeenk FWJM, Smeele IJ, Van Schayck CP. Overtreatment with inhaled corticosteroids and diagnostic problems in primary care patients, an exploratory study. Fam Pract. 2008;25(2):86–91.
Griffith MF, Feemster LC, Zeliadt SB, et al. Overuse and misuse of inhaled corticosteroids among veterans with COPD: a cross-sectional study evaluating targets for de-implementation. J Gen Intern Med. 2020;35(3):679–86.
Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;10(3):CD010115.
Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176(2):162–6.
Lin J, Li Y, Tian H, et al. Costs and health care resource utilization among chronic obstructive pulmonary disease patients with newly acquired pneumonia. Clinicoecon Outcomes Res. 2014;6:349–56.
Ryan M, Suaya JA, Chapman JD, Stason WB, Shepard DS, Thomas CP. Incidence and cost of pneumonia in older adults with COPD in the United States. PLoS One. 2013;8(10):e75887.
Palli SR, Frazer M, DuCharme M, Buikema AR, Anderson AJ, Franchino-Elder J. Differences in real-world health and economic outcomes among patients with COPD treated with combination tiotropium/olodaterol versus triple therapy. J Manag Care Spec Pharm. 2020;26(10):1363–74.
Mammen MJ, Lloyd DR, Kumar S, et al. Triple therapy versus dual or monotherapy with long-acting bronchodilators for COPD: a systematic review and meta-analysis. Ann Am Thorac Soc. 2020;17(10):1308–10.
Rogliani P, Ritondo BL, Gabriele M, Cazzola M, Calzetta L. Optimizing de-escalation of inhaled corticosteroids in COPD: a systematic review of real-world findings. Expert Rev Clin Pharmacol. 2020;13:977–90.
Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55(6):2000351.
Beeh KM, Derom E, Echave-Sustaeta J, et al. The lung function profile of once-daily tiotropium and olodaterol via Respimat® is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler® (ENERGITO® study). Int J Chron Obstruct Pulmon Dis. 2016;11:193–205.
Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–22.
Tariq SM, Thomas EC. Maintenance therapy in COPD: time to phase out ICS and switch to the new LAMA/LABA inhalers? Int J Chron Obstruct Pulmon Dis. 2017;12:1877–82.
Stephenson JJ, Wertz D, Gu T, Patel J, Dalal AA. Clinical and economic burden of dyspnea and other COPD symptoms in a managed care setting. Int J Chron Obstruct Pulmon Dis. 2017;12:1947–59.
Trudo F, Kern DM, Davis JR, et al. Comparative effectiveness of budesonide/formoterol combination and tiotropium bromide among COPD patients new to these controller treatments. Int J Chron Obstruct Pulmon Dis. 2015;10:2055–66.
Ke X, Marvel J, Yu T-C, et al. Impact of lung function on exacerbations, health care utilization, and costs among patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1689–703.
Kern DM, Williams SA, Tunceli O, et al. A US database study characterizing patients initiating a budesonide-formoterol combination versus tiotropium bromide as initial maintenance therapy for chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:775–83.
Wallace AE, Kaila S, Bayer V, et al. Health care resource utilization and exacerbation rates in patients with COPD stratified by disease severity in a commercially insured population. J Manag Care Spec Pharm. 2019;25(2):205–17.
Suissa S, Dell’Aniello S, Ernst P. Long-acting bronchodilator initiation in COPD and the risk of adverse cardiopulmonary events: a population-based comparative safety study. Chest. 2017;151(1):60–7.
Desai RJ, Rothman KJ, Bateman BT, Hernandez-Diaz S, Huybrechts KF. A propensity-score-based fine stratification approach for confounding adjustment when exposure is infrequent. Epidemiology. 2017;28(2):249–57.
Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–34.
Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–94.
Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–26.
Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48(4):1030–9.
Samp JC, Joo MJ, Schumock GT, Calip GS, Pickard AS, Lee TA. Comparative effectiveness of long-acting beta2-agonist combined with a long-acting muscarinic antagonist or inhaled corticosteroid in chronic obstructive pulmonary disease. Pharmacotherapy. 2017;37(4):447–55.
Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158–65.
Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;6(11):855–62.
Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–80.
Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48.
Thornton Snider J, Luna Y, Wong KS, et al. Inhaled corticosteroids and the risk of pneumonia in Medicare patients with COPD. Curr Med Res Opin. 2012;28(12):1959–67.
Mullerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106(8):1124–33.
Finney LJ, Padmanaban V, Todd S, Ahmed N, Sarah LE, Mallia P. Validity of the diagnosis of pneumonia in hospitalised patients with COPD. ERJ Open Res. 2019;5(2):00031–2019.
Moretz C, Sharpsten L, Bengtson LG, et al. Real-world effectiveness of umeclidinium/vilanterol versus fluticasone propionate/salmeterol as initial maintenance therapy for chronic obstructive pulmonary disease (COPD): a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:1721–37.
Acknowledgements
Funding
Support for this project and the journal’s Open Access Fee were funded by Boehringer Ingelheim International GmbH. No Rapid Service Fee was received by the journal for the publication of this article.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim and provided by Vicki Cronin of MediTech Media, under the authors’ conceptual direction and based on feedback from the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
This manuscript is based on work previously presented as a poster at the 2020 American Thoracic Society International Conference.
Disclosures
Jennifer K. Quint’s research group has received funds via Imperial Consultants. She has received grants from Bayer, Boehringer Ingelheim, Imperial Consultants, Insmed, and GlaxoSmithKline; and personal fees from Bayer, Boehringer Ingelheim, Insmed, and GlaxoSmithKline. Jukka Montonen, Laura Wallace and Alberto de la Hoz are employees of Boehringer Ingelheim. Xintong He and Leslie Koerner are employees of HealthCore Inc, which received funding from Boehringer Ingelheim to conduct the study. Daina B. Esposito received funding from HealthCore Inc for participation in this work. Marc Miravitlles has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Grifols, Menarini, Novartis, Rovi, Sandoz, and Zambon; consulting fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, CSL Behring, Gebro Pharma, Ferrer, GlaxoSmithKline, Grifols, Kamada, Laboratorios Esteve, Mereo Biopharma, Novartis, pH Pharma, Sanofi, Spin Therapeutics, TEVA, and Verona Pharma; and research grants from GlaxoSmithKline and Grifols.
Compliance with Ethics Guidelines
This study was conducted using administrative claims in the form of a limited data set pursuant to data agreements between HealthCore and participating health plans in compliance with the Health Insurance Portability and Accountability Act. The study did not require ethics committee approval, nor were subjects required to provide informed consent.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available, in compliance with the US Health Insurance Portability and Accountability Act Privacy Rule (45 CFR Part 160 and Subparts A and E of Part 164), which states that identifiable protected health information data should not be accessed, used or disclosed unless a specific waiver of authorization is granted. HealthCore accessed the data in a manner that complies with applicable federal and state laws and regulations, including those related to the privacy and security of individually identifiable health information.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Quint, J.K., Montonen, J., Esposito, D.B. et al. Effectiveness and Safety of COPD Maintenance Therapy with Tiotropium/Olodaterol versus LABA/ICS in a US Claims Database. Adv Ther 38, 2249–2270 (2021). https://doi.org/10.1007/s12325-021-01646-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01646-5