Abstract
Introduction
The first long-acting release (LAR) formulation of octreotide was marketed in France in the late 1990s. An injectable formulation of Sandostatin LAR® (Novartis SAS) with a new diluent has been developed to facilitate its preparation and administration and to improve its use in practice.
Methods
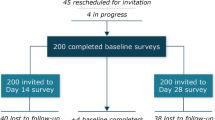
We conducted an observational, cross-sectional and multicenter study in France whose main outcome was to compare nurses’ satisfaction with the preparation and administration of both previous and new formulations of octreotide LAR. Secondary outcomes included assessment of patient satisfaction (quality of life and pain felt during the injection) and product tolerance. Data were collected at two time points (one for the first formulation group and one for the second formulation group) through paper questionnaires administered to physicians, patients and nurses including a visual analog scale (VAS) from 0 (unsatisfied) to 10 (very satisfied).
Results
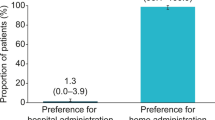
Results showed that overall nurse satisfaction improved from 5.3 (95% CI 4.9–5.8) with the previous formulation to 7.5 (95% CI 7–7.9) with the new formulation (p < 0.0001). Regarding secondary outcomes, the simplicity of the injection increased (84% for the previous formulation and 94% for the new formulation) and the purge problem disappeared (36% for the previous formulation and 4% for the new formulation).
Conclusion
The improvement due to the new formulation of Sandostatin LAR® was reported in terms of handling, ease of use and overall nurse satisfaction. The new formulation greatly reduced treatment administration problems associated with the previous formulation, while maintaining low injection site pain and an equivalent safety profile in both indications.



Similar content being viewed by others
References
(HAS), H. A. de S. (2019) Avis Sandostatine LAR. https://www.has-sante.fr/upload/docs/evamed/CT-15938_SANDOSTATINE_LP_QD_INS_Avis1_CT15938.pdf.
Adelman D, et al. Evaluation of nurse preferences between the lanreotide autogel new syringe and the octreotide long-acting release syringe: an international simulated-use study (PRESTO). Adv Therapy. 2020. https://doi.org/10.1007/s12325-020-01255-8.
Anthony L, Freda PU. From somatostatin to octreotide LAR: Evolution of a somatostatin analogue. Curr Med Res Opin. 2009. https://doi.org/10.1185/03007990903328959.
Carlson J, et al. Rituximab for subcutaneous delivery: clinical management principles from a nursing perspective. Int J Nurs Pract. 2015. https://doi.org/10.1111/ijn.12413.
Chanson P, et al. Acromegaly. Best Pract Res Clin Endocrinol Metab. 2009. https://doi.org/10.1016/j.beem.2009.05.010.
Chanson P. Medical treatment of acromegaly with dopamine agonists or somatostatin analogs. Neuroendocrinology. 2016. https://doi.org/10.1159/000377704.
Crespo I, Valassi E, Webb SM. Update on quality of life in patients with acromegaly. Pituitary. 2017. https://doi.org/10.1007/s11102-016-0761-y.
De Mestier L, et al. Thésaurus national de cancérologie digestive (TNCD). Digestive Neuroendocrine neoplasms (NEN): French intergroup clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig Liver Dis. 2020. https://doi.org/10.1016/j.dld.2020.02.011.
Dharan SS, Kamaruddin NA. Successful primary medical therapy with somatostatin receptor ligand in acromegaly with thyroid cancer. J ASEAN Fed Endocr Soc. 2017. https://doi.org/10.15605/jafes.032.02.12.
EuroQol–a new facility for the measurement of health-related quality of life. Health policy. 1990. https://doi.org/10.1016/0168-8510(90)90421-9.
Fattah S, Brayden DJ. Progress in the formulation and delivery of somatostatin analogs for acromegaly. Ther Deliv. 2017. https://doi.org/10.4155/tde-2017-0064.
Fenwick S, Thakur K, Munro D. Nurse and patient perceptions and preferences for subcutaneous autoinjectors for inflammatory joint or bowel disease: findings from a European Survey. Rheumatol Therapy. 2019. https://doi.org/10.1007/s40744-019-0144-8.
Gilroy JJ, James RA. Optimizing somatostatin analog therapy in acromegaly: Long-acting formulations. Treat Endocrinol. 2002. https://doi.org/10.2165/00024677-200201030-00002.
Lenderking WR, et al. The reliability and validity of the impact on lifestyle questionnaire in patients with acromegaly. Value Health. 2000. https://doi.org/10.1046/j.1524-4733.2000.34003.x.
Maher K, et al. Improvements for patients and nurses using 2.5 ml prefilled syringes as the vehicle solution for suspension of Sandostatin LAR® microspheres. In: 8th European Congress of Endocrinology incorporating the British Endocrine Societies. Glasgow; 2006.
Melmed S, et al. A Consensus Statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018. https://doi.org/10.1038/s41574-018-0058-5.
Plunkett C, Barkan AL. The care continuum in acromegaly: How patients, nurses, and physicians can collaborate for successful treatment experiences. Patient Preference Adherence. 2019. https://doi.org/10.2147/PPA.S84887.
Shogbon AO, et al. Nurses’ perceptions and satisfaction with the use of insulin pen devices compared with insulin vial and syringes in an inpatient setting. Diabet Technol Ther. 2014. https://doi.org/10.1089/dia.2014.0072.
Singh S, et al. Patient-reported burden of a neuroendocrine tumor (NET) diagnosis: results from the first global survey of patients with NETs. J Global Oncol. 2017. https://doi.org/10.1200/jgo.2015.002980.
Strasburger CJ, et al. Patient-reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol. 2016. https://doi.org/10.1530/EJE-15-1042.
Tischer B, Mehl A. Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Preference Adherence. 2018. https://doi.org/10.2147/PPA.S169339.
Yang LPH, Keating GM. Octreotide long-acting release (LAR): a review of its use in the management of acromegaly. Drugs. 2010. https://doi.org/10.2165/11204510-000000000-00000.
Acknowledgements
We thank study participants for their involvement in the study. Participating physicians: Abakar Mahamat, A; Ajzenberg, C; Albarel Loy, F; Assenat, E; Baconnier, M; Bihan, H; Bouhier Leporrier, K; Bourcigaux, N; Brezault Bonnet, C; Brue, T; Cadiot, G; Caulet, M; Chabre, O; Chanson, P; Coriat, R; Dadamessi, I; Dauvois, B; Deblock, M; Decoudier, B; Delemer, B; Desailloud, R; Desauw, C; Desrame, J; Di Fiore, F; Dominguez, S; Donadille, B; Donnet, J; Dourthe, L; Etienne, P; Fagour, C; Fonck, M; Garnier Tixidre, C; Goichot, B; Guedj, A; Guimbaud, R; Hassler, P; Hentic Dhome, O; Iwanicki Caron, I; Jeandidier Gauthier, N; Joly, J; Jublanc, C; Klein, M; Labourey, J; Lagasse, P; Lecomte, T; Leyronnas, C; Locher, C; Maire, F; Manfredi, S; Mary, F; Mazard, T; Merlen Kosydar, E; Metges, J; Michaud Herbst, A; Mineur, L; Mouysset, J; Nadalon Chambrier, S; Nahon, S; Nguyen Tan Hon, T; Niccoli, P; Obled, S; Pascal-Vigneron, V; Penfornis, A; Poupardin Moulin, C;Prevost, J; Raingeard, I; Raverot, G; Requeda, E; Rodien, P; Rodien, P; Samalin, E; Schillo, F; Smith, D; Sobhani, I; Sonnet, E; Tabarin, A; Terrebonne, E; Thomas, J; Touraine, P; Velayoudom, F; Verier Mine, O; Verveur, C; Villing, A; Yakendji, K; Zannetti, A. Participating Nurses: Abassebay, S; Aguililla, E; Alazaud, E; Anglade, V; Anglio, M; Aubriot, C; Audebert, C; Auger, S; Badani, M; Balligand, P; Barriere, F; Belle, S; Benamor, D; Berenger, C; Berger, L; Beroudiaux, B; Bertin, A; Bertin, C; Bidan, C; Bodet, C; Bonardi, O; Bonneau, P; Bonnefe, B; Bonnotte, O; Boucher, I; Boulanger, C; Brasseur, G; Braun/Reuter, B; Buguet, C; Cados, P; Cambot, I; Capelle, J; Cappelle, L; Caruso, F; Chadefaud, S; Chanu, P; Chatreaux, S; Chavanon–Vidal, C; Cheron, L; Chesini, M; Claerebout, C; Cocaign-Lepelletier, C; Coillot, C; Connant-Rault, N; Consigny, K; Costa, C; Couraceiro, A; Courbet, A; Courtois, F; Coutin, C; Cugnenc, V; Cyrille, S; Dalla-Riva, M; Dartier, E; De Brito, M; De Luca, L; Deboudt, C; Debs, M; Delbe, M; Delbé, P; Deodato, G; Deporcq, C; Derez, C; Desseaux, I; Didier, J; Donnet, A; Doridot, L; Douay, B; Druais, S; Dubarry, E; Dubois, G;Dudel, I; Duhoo, L; Dutartre-Lacroix, C; Edeline, B; Elixee, C; Emiliani, S; Eymann, V; Eymard, I; Faichaud, J; Febvre, M; Fligeat, R; Flippe, C; Foret, C; Forget, E; Fouliard, L; Frerot, V; Friderich, N; Galpin, V; Gatard, F; Gatumel, J; Gentil, S; Giboulet, A; Girot, O; Golebiewski, C; Gotni, F; "Grandgirard, C; "Guillou, V; Guinault, P; Hamann-Gresset, E; Harant, A; Heliot, M; Henry, C; Hergas, E; Hesse, M; Hubert-Piesseau, M; Jean, T; Jean-Jean, P; Jeanmarie, M; Karamane, A; Klisz, F; Korczynski, A; Krajcovic, J; Labrunie, L; Langlet, B; Lanu, S; Laplaze, C; L'arvor, A; Laurent–Chapuis, L; Laurent, C; Laval, C; Le Bigot, N; Le Faucheur, A; Lê Quang, S; Lebreton, P; Leger, V; Lelong, L; Leon, A; Leroy, P; Lithard, C; Lohou, S; Lorber, S; Lowes, S; Maciejewski, K; Mack, I; Maisonneuve, F; Marichal-Abrial, C; Maroszak (), -; Martial, P; Martin, B; Meline, E; Meuret (), P; Mevel, S; Moisset, C; Mongin, B; Montaigne, C; Moreau, M; Moreno, C; Mouffok, M; Mounsif, H; Musard, F; Nancey, E; Oliveira Da Costa, A; Orsini, D; Pananceau, L; Parcy, N; "Paubet, P; Paudert, G; Pelletane, C; Perard, V; Perlin, A; Perriguey,; Petrovic, P; Philippe, S; Pierry, C; Pittet, F; Pittet, M; Pomarico, A; Ponsan, J; Prevost, M; Proux, M; Prunier, N; Rambeaud, P; Rannou, C; Redo, A; Retournay, E; Reynaud, J; Reynaud, R; Rigollot, I; Robardet, C; Rochdy, E; Rosamond, V; Rouby, M; Rouille, J; Roumeas, E; Rozencwajg, L; Runigo, C; Sadrant, J; Salingue, A; Sam Bassoum, R; Saran, F; Sayah, N; Sche R, J; Schelpe, G; Sevin-Roux, C; Sighele, C; Sigonney, A; Silvin, M; Simba, P; Solagna–Fernandez, A; Solvar, M; Sottana, D; Tailllandier, V; Tessier, J; Travers, J; Travert, C; Troufflard, A; Turbiarz, S; Valmorin, C; Vandevelde, M; Vedel, A; Verite, S; Vesta, M; Veyssiere, E; Vidal, T; Walczak, S.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Novartis Pharma.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
The authors received writing/editorial support in the preparation of this manuscript provided by Ilaria Montagni-Duchemin, PhD, of IMD-SMW, funded by Novartis, Inc. Medical editorial assistance was also provided by Kappa Santé.
Disclosures
Guillaume Cadiot has served on an advisory board for Novartis, Ipsen, AAA and Keocyt. Brigitte Delemer has served on advisory boards for novartis, ipsen, pfizer, sanofi and novo-nordisk. Romain Coriat received consultancy fees from Amgen, Keocyt, AAA, Novartis, Ipsen, Bayer and Servier and grant support from Ipsen, Amgen and the Fondation-Université de Paris "sauvez la vie." Denis Smith received consultancy fees from Novartis, Ipsen and AAA. Iradj Sobhani received investigator fees from Novartis and Ipsen. Benedicte Decoudier received consultancy fees from Novartis. Gerald Raverot received consultancy fees from Novartis and Pfizer, investigator fees from Novartis, Ipsen and Pfizer and grant support from Ipsen and Novartis. Alexandre Santos and Ségolène Biso-Locard are employees of Novartis. Thierry Nguyen-Tan-Hon, Frank Schillo, Isabelle Raingeard and Pierre-Luc Etienne have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved on September 6, 2012, by the French advisory committee on the processing of health research information (Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé, CCTIRS). The study was conducted in compliance with the recommendations of the Helsinki Declaration of 1964 and its later amendments. Patients were asked to give their oral consent to participate in the study after having received and read the information note presented by the investigating physician.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12582029.
Rights and permissions
About this article
Cite this article
Delemer, B., Nguyen-Tan-Hon, T., Coriat, R. et al. Evaluation of Nurses’ and Patients’ Overall Satisfaction with New and Previous Formulations of Octreotide Long-acting Release (Sandostatin LAR®): A French Observational Study. Adv Ther 37, 3901–3915 (2020). https://doi.org/10.1007/s12325-020-01429-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01429-4