Abstract
The prevalence of non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D) is increasing. As a strong association between these two diseases exist, it is unsurprising that the number of patients with coexisting NAFLD and T2D is also increasing. These patients display a deleterious metabolic profile (e.g. hypertriglyceridemia), and increased mortality rates relative to those with only NAFLD or T2D in isolation; therefore, effective treatment strategies are required. Here we review the available intervention studies that have investigated the effects of changes in lifestyle (diet and exercise/physical activity) on NAFLD in patients with both NAFLD and T2D. On the basis of the available evidence, it appears that the addition of any kind of exercise (i.e. resistance, aerobic, or high-intensity intermittent exercise) is beneficial for patients with both NAFLD and T2D. These effects appear to occur independently of changes in body weight. Hypocaloric diets leading to weight loss are also effective in improving metabolic parameters in patients with both NAFLD and T2D, with data indicating that ~ 7–10% weight loss is required in order to observe beneficial effects. It is unclear if multidisciplinary interventions incorporating changes in both diet and physical activity levels are a more effective treatment strategy in this population than diet or exercise interventions in isolation. In conclusion, it is clear that lifestyle interventions are an effective treatment strategy in patients with both NAFLD and T2D, although further research is required to optimise these interventions and determine their scalability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Globally, the number of patients with coexisting non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes (T2D) is increasing. As these patients display a deleterious metabolic profile (e.g. hypertriglyceridemia), and increased mortality rates relative to those with NAFLD or T2D in isolation, effective treatment strategies are urgently required. |
At present, there exists no approved pharmacological treatment for NAFLD, and as such lifestyle interventions represent the recommended management strategy. |
On the basis of the available evidence, it appears that both increasing physical activity levels and adopting a hypocaloric diet reduce intrahepatic triacylglycerol (IHTAG) content and improve glycaemic control/insulin sensitivity in patients with both NAFLD and T2D. |
Future research is required to establish the cost-effectiveness of lifestyle interventions and the feasibility of delivering such interventions within a clinical setting. |
Introduction
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of liver-related conditions, ranging from steatosis (characterised by an accumulation of intrahepatic triacylglycerol (IHTAG)) to non-alcoholic steatohepatitis (NASH) (characterised by the presence of hepatocyte injury and fibrosis), cirrhosis (accumulation of scar tissue), and hepatocellular carcinoma [1].
Diagnosing NAFLD
The early stages of NAFLD are typically asymptomatic with investigations only performed following an incidental finding of abnormal liver enzymes (i.e. raised plasma alanine aminotransferase (ALT), aspartate transaminase (AST), and/or gamma-glutamyltransferase (γGT)) [2]. However, the vast majority of patients with NAFLD (~ 80%) exhibit liver enzymes within the normal range [3]; liver enzymes may fluctuate dependent on NAFLD stage [4]. Plasma ALT and AST have been shown, by some, to not correlate with histological assessments of NAFLD, and are clinically poor markers of the presence and severity of NAFLD [4, 5], thus, a large proportion of patients with NAFLD may remain undiagnosed. The “gold standard” diagnosis method is liver biopsy, whereby NAFLD is defined by the presence of IHTAG in more than 5% of hepatocytes [6]. Liver biopsy is also the only method which is able to fully determine the stage and severity of NAFLD as it enables the assessment of pathological features such as hepatocyte ballooning, lobular inflammation, and fibrosis [7]. However, biopsies are expensive, invasive, and potentially dangerous, limiting their use in screening or monitoring of patients. Furthermore, biopsies only obtain a relatively small quantity of tissue (~ 1:50,000 relative to total liver size), and are therefore subject to sampling variability, which may potentially result in misdiagnosis and staging inaccuracies [8].
NAFLD can also be investigated non-invasively through the use of imaging techniques and/or predictive equations using biomarkers. Ultrasound is the most common imaging modality used to evaluate hepatic steatosis because of its low cost, safety, and availability; ultrasound represents the first line in diagnosing NAFLD [9]. The severity of NAFLD is usually graded clinically using a four-point scoring system: normal (grade 0), mild (grade 1), moderate (grade 2), and severe (grade 3) [10]. The diagnostic performance of ultrasound has been reported to vary according to the degree of hepatic steatosis. In patients without coexisting liver disease, ultrasound offers an accurate diagnosis of moderate-to-severe hepatic steatosis (i.e. IHTAG ≥ 30%) [10]. In contrast, when comparing ultrasound to other imaging techniques and histopathologic assessments of steatosis, ultrasound has been shown to be less accurate in detecting hepatic steatosis than magnetic resonance imaging/spectroscopy (MRI/S) when all degrees of steatosis were considered (IHTAG ranging from 0% to 80%) [11]. Regarding MRI/S, NAFLD is associated with a proton density fat fraction (defined as the amount of protons bound to fat divided by the amount of all protons in the liver, including those bound to fat and water) greater than 5.56%, a figure which is based on the 95th percentile of IHTAG observed in the Dallas Heart Study [12]. Predictive models and biomarkers utilising modern technologies such as genetic testing and ‘omics’ methodologies are currently being investigated [13, 14], although the efficacy of using these approaches to screen for NAFLD is limited due to a number of factors including cost and a lack of validation.
NAFLD and Type 2 Diabetes
By definition NAFLD is only diagnosed when one or more of the pathological features are reported in individuals who do not consume excessive amounts of alcohol (i.e. more than 20 g/day for women and more than 30 g/day for men), and in the absence of other liver aetiologies [2, 6]. NAFLD is currently the most prevalent form of liver disease in the world, thought to affect ~ 6–50% of the adult population, dependent on the population studied and the method of assessment [15, 16]. In obesity the prevalence of NAFLD increases to ~ 50–75% [17,18,19,20,21], highlighting the relationship between IHTAG and total adiposity. IHTAG is also correlated with other features of the metabolic syndrome [22], leading to NAFLD being referred to as the “hepatic manifestation of the metabolic syndrome” [23]. As obesity is a common risk factor for both type 2 diabetes (T2D) and NAFLD, it is unsurprising that there is a strong association between NAFLD and T2D prevalence, which has been reported to be as high as 87% [24]. There is much deliberation regarding the pathogenesis of these two interrelated conditions, and there appears to exist a complex bidirectional relationship whereby presence of one drives progression of the other [25, 26]. What is known is that patients with T2D have elevated IHTAG when compared to age-, gender-, and body mass index (BMI)-matched subjects without T2D [27]. Furthermore, the coexistence of NAFLD and T2D is also associated with a poorer metabolic profile (i.e. inferior glycaemic control, dyslipidaemia, and increased risk of cardiovascular disease), alongside increased microvascular complications, NAFLD progression, and total mortality when compared to NAFLD or T2D in isolation [16, 28]. Coexisting NAFLD and T2D may also increase the insulin requirements of a patient with T2D undergoing insulin therapy [29], which may have implications for the regulation of body weight [28].
Lifestyle Interventions in Patients with NAFLD
At present there exists no approved pharmacological treatment for NAFLD, and as such lifestyle interventions represent the recommended management strategy. However, pharmacological therapies commonly prescribed for T2D may influence NAFLD, with drugs that induce weight loss (e.g. liraglutide and orlistat) showing particular promise (as reviewed in [16, 28, 30]). The effectiveness of lifestyle interventions on improving various markers of NAFLD (i.e. reduction of circulating liver enzymes, steatosis, and presence of NASH) was recently demonstrated in a systematic review of 22 randomised clinical trials with 2588 patients with NAFLD [31]. However, less is known regarding the effect of lifestyle interventions in patients with both NAFLD and T2D. Here, we provide a narrative review on the effects of lifestyle interventions on NAFLD from studies which have included patients with both NAFLD and T2D. For this purpose “lifestyle interventions” will refer to any studies where patients with both NAFLD and T2D underwent controlled interventions designed to alter habitual diet and physical activity levels, or where behavioural interventions were employed to actively encourage participants to make lifestyle changes. Studies were only included where the proportions of participants with both NAFLD and T2D were clearly defined, or where baseline participant characteristics indicated that a proportion of participants would cross the diagnostic thresholds for both NAFLD and T2D. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Physical Activity and Exercise Interventions
Both NAFLD and T2D are associated with reduced physical activity levels and a sedentary lifestyle [32,33,34]. Results from meta-analysis have shown that exercise interventions are associated with a reduction in IHTAG, a response that appears independent of weight loss [35, 36].
Aerobic vs. Resistance Exercise Interventions
The majority of studies investigating exercise interventions and NAFLD have involved aerobic exercise [35, 36]. However, Hallsworth et al. [37] randomised 19 people with NAFLD to a resistance exercise program or standard care for 8 weeks (Table 1). Resistance exercise involved three sessions a week where participants underwent a whole-body workout using machine-based exercises performed at 50–70% of their one-repetition maximum. At the end of 8 weeks, BMI remained unaltered in both groups, but there was a significant reduction in IHTAG in the exercise group only, which was associated with an improvement in postprandial glycaemic control. This suggests that resistance exercise may have a positive influence on decreasing IHTAG content. When comparing exercise modalities, Bacchi et al. [38] found 4 months of resistance training to be equally effective in reducing IHTAG and increasing insulin sensitivity as aerobic exercise training among patients with both NAFLD and T2D (Table 1). Thus, it would appear that simply increasing physical activity, through either aerobic or resistance exercise, reduces IHTAG and positively influences glycaemic control in patients with coexisting NAFLD and T2D.
High-Intensity Intermittent Training
The most commonly cited barrier to performing physical activity/exercise is a “lack of time” [39] and, as such, short-duration high-intensity intermittent training (HIIT) has emerged as a potential strategy for improving metabolic health. Alongside being more time-efficient, HIIT has also been shown to be more enjoyable, for some, than moderate continuous exercise [40, 41]. Cassidy et al. [42] randomised 23 patients with T2D to a 12-week HIIT intervention or standard care. HIIT involved three sessions a week of cycling exercise where participants performed five intervals of “very hard” cycling separated by 90 s of recovery (Table 1). The durations of the “very hard” cycling were progressive across the intervention and ranged from ~ 2 min to ~ 4 min. Body weight was not significantly influenced by the intervention, but IHTAG displayed a relative reduction of ~ 39% (from ~ 6.9% to ~ 4.2% in absolute terms) along with improvements in cardiac function and glycaemic control, whilst no changes in these parameters were found in the control group. Reductions in IHTAG following HIIT have also been observed by others, alongside reductions in circulating ALT, AST and γGT, and improvements in markers of insulin sensitivity [43,44,45] (Table 1), indicating that HIIT may be an effective intervention for those with both NAFLD and T2D. However, for those new to exercise it may be challenging to achieve the exercise intensities necessary to elicit benefits from HIIT; therefore, interventions may need to be adapted according to the patients’ abilities.
Low-Intensity and Web-Based Exercise Interventions
Notably, the majority of studies included in a meta-analysis by Sargeant et al. [36] were classified as “high-intensity” exercise interventions. It is plausible that the ability to perform physical activity or exercise sessions may be compromised in patients with more advanced NAFLD and/or T2D, which may limit the generalisability of physical activity and exercise interventions. This is pertinent not only to interventions focused solely on increasing physical activity but also on those employing multidisciplinary interventions. Evidence does exist to demonstrate that low-intensity exercise reduces IHTAG content [46], although in this study low-intensity exercise involved continuous cycling and brisk walking which may still be challenging for some to undertake. Furthermore, participants in this study were recruited on the basis of BMI, and not presence of NAFLD or T2D, and it is unclear if those with both NAFLD and T2D would demonstrate similar responses. Further research is required to corroborate this finding and establish whether interventions of even lower-intensity activities (e.g. interrupting sitting time with periods of standing) influence markers of NAFLD in patient populations.
Whilst certain exercise interventions may require specialist staff and equipment, a recent study by Huber and colleagues demonstrates the utility of a web-based intervention, where exercise was designed to be performed in the domestic environment, and participants received support via an online feedback platform [47]. Individuals who undertook the 8-week intervention lost a small but significant amount of body weight (~ 0.9%) and demonstrated improvements in a number of metabolic risk factors, including surrogate markers of hepatic steatosis and fibrosis (Table 1). A number of these clinical improvements were still apparent at a 20-week follow-up visit. This feasibility study demonstrates the efficacy of adopting a web-based intervention, a strategy which may reduce the burden placed on resources (specialist staff and equipment) of traditional exercise interventions.
Dietary Interventions
Dietary intervention studies include those which have altered total energy intake or dietary macronutrient composition, along with those which have provided participants with dietary supplements alongside their habitual diets.
Hypocaloric Diets
Hypocaloric diets (i.e. diets providing a lower number of calories than that required to maintain energy balance) consistently lower IHTAG content and improve markers of insulin sensitivity/glycaemic control. For example, Lim et al. [48] investigated the effects of an 8-week hypocaloric diet (~ 600 kcal/day) in 11 patients with T2D. The diet involved the consumption of liquid formula (46% total energy (TE) carbohydrate, 33% TE protein, and 20% TE fat) supplemented with vegetables. The authors observed a significant reduction in plasma glucose after 1 week of the intervention (from 9.2 ± 0.4 to 5.9 ± 0.4 mmol/L) which was maintained at week 4 and similar to non-diabetic controls (Table 2). Reductions in endogenous glucose production and improvements in hepatic insulin sensitivity were also apparent after 1 week, which occurred alongside a ~ 30% reduction in IHTAG. By the end of the 8-week intervention period participants had decreased body weight by ~ 15% and IHTAG had decreased by ~ 70% [48] (Table 2). These dramatic results were repeated in the Diabetes Remission Clinical Trial (DiRECT) where it was demonstrated that in individuals diagnosed with T2D for fewer than 6 years a hypocaloric diet (~ 825–853 kcal/day) restored normal glycaemic control [49]. The diet involved the consumption of liquid formula (59% TE carbohydrate, 13% TE fat, 26% TE protein) which was consumed for 3–5 months. This was then followed by a structured period of food reintroduction (2–8 weeks), followed by monthly visits aimed at supporting maintenance of weight loss until 12 months. In a geographically determined subgroup of the DiRECT cohort Taylor et al. [50] examined IHTAG, very low density lipoprotein triacylglycerol (VLDL-TAG) secretion, pancreas fat content, and β-cell function (Table 2). Participants were separated into subgroups based on whether or not they demonstrated diabetes remission (i.e. HbA1c < 6.5%, blood glucose < 7.0 mmol/L, and off any anti-diabetes medication for at least 2 months prior to investigations) following the intervention (i.e. “responders” and “non-responders”). IHTAG content decreased to a similar extent in both groups (~ 13% vs. ~ 12%, for responders vs. non-responders respectively), whereas VLDL-TAG secretion was only significantly reduced in responders. The authors hypothesise that the increased VLDL-TAG secretion rate in non-responders may be attributable to reductions in hepatic insulin sensitivity, which may increase β-cell exposure to fat, negatively influencing pancreatic fat accumulation and β-cell lipotoxicity. However, changes in total plasma TAG and pancreas fat were similar between responders and non-responders. Furthermore, an earlier study by the same group found similar reductions in IHTAG and VLDL-TAG secretion in those who achieved a reduction in fasting blood glucose (< 7.0 mmol/L) in response to a hypocaloric diet (i.e. “responders”) compared to those who did not (i.e. “non-responders”) [51].
Weight Loss and Metabolic Improvements
Regarding the amount of weight loss required to achieve metabolic benefits, Petersen et al. [52] investigated the effect of a hypocaloric (~ 1200 kcal/day) liquid formula diet (50% TE carbohydrate, 43% TE protein, 3% TE fat) supplemented with fruits and vegetables for 3–12 weeks, in eight individuals with obesity and T2D (Table 2). The hypocaloric diet was terminated when participants returned to normoglycaemia. The authors report that normoglycaemia was attained at ~ 8% weight loss, which occurred alongside a reduction in IHTAG of ~ 81% (from ~ 12% to ~ 2% in absolute terms), reduced endogenous glucose production, and increased hepatic insulin sensitivity. No change in peripheral insulin sensitivity was observed. This finding suggests that in response to weight loss, changes at the level of the liver induce whole-body improvements in glucose metabolism. Evidence also suggests that a dose–response relationship exists in regards to weight loss and metabolic improvements; Vilar-Gomez et al. [53] reported that compared to standard care, 1 year of low-carbohydrate ketogenic diet (i.e. less than 30 g carbohydrate/day, 1.5 g protein per kg, and no restrictions on fat) improved markers of NAFLD, HbA1c, fasting plasma glucose, fasting plasma insulin, homeostatic model assessment for insulin resistance (HOMA-IR), and plasma TAG (Table 2). Approximately 79% of patients who undertook the ketogenic diet decreased body weight by 5–10%, with just over half the group decreasing by greater than 10%; the patients who lost more than 10% of their body weight demonstrated the greatest improvements in the surrogate markers of NAFLD. Combined, these data highlight the importance of weight loss in managing NAFLD and T2D.
Influence of Macronutrient Composition on NAFLD and T2D
At present, the Mediterranean dietary pattern is recommended for the management of NAFLD [6]. However, it remains to be determined if macronutrient composition influences the responsiveness of individuals with both NAFLD and T2D to a hypocaloric diet. Supplementing with n-3 polyunsaturated fatty acids (PUFA) may reduce IHTAG in patients with NAFLD [54]. However, in a paired liver biopsy study of patients with T2D, Dasarathy et al. [55] reported no significant change in histological markers of steatosis or NASH in response to 48 weeks of daily supplementation with n-3 fatty acids [i.e. eicosapentaenoic acid (EPA; 2160 mg/day) and docosahexaenoic acid (DHA; 1440 mg/day)] compared to placebo (Table 2). Body weight was similar in both the n-3 PUFA and placebo groups, and was not affected by the intervention. Alongside no difference in histological parameters, the authors also noted an increased fasting plasma glucose concentration and HbA1c in the n-3 PUFA group, but not the placebo group (Table 2). Thus, it appears that n-3 fatty acid supplementation may not be beneficial in patients with coexisting NASH and T2D. However, other aspects of lifestyle that may be a confounder of results (i.e. habitual diet and physical activity levels) were not assessed in this study, and neither were plasma levels of EPA and DHA, making it challenging to ascertain adherence to the intervention.
Multidisciplinary Interventions
The majority of studies investigating the impact of lifestyle interventions on NAFLD and T2D have employed a multidisciplinary approach where hypocaloric diets (of varying macronutrient compositions) have been prescribed for weight loss, alongside increased physical activity or exercise training regimens.
Hypocaloric Diet and Physical Activity Interventions
When analysing data obtained from the CURIAMO project [56], Reginato et al. [57] reported that a 13-week structured, exercise and nutritional education program improved surrogate indices of NAFLD (i.e. visceral adiposity index, fatty liver index, liver fat equation, and the TAG and glucose index) (Table 3). The exercise program consisted of both aerobic and resistance exercise and was supervised by a professional in exercise sciences, and the nutritional education program consisted of four sessions aimed at improving Mediterranean diet score and was led by a dietitian. Improvements in markers of NAFLD and metabolic health were also reported by Sun et al. [58] who observed reductions in BMI, waist circumference, ALT, and HOMA-IR in patients who were provided with an individually tailored diet and encouraged to increase their physical activity for 12 months (Table 3). Furthermore, Thomas et al. [59] reported a significant reduction in body weight of ~ 3.5 kg (~ 4% in relative terms) and a non-significant reduction in IHTAG of ~ 40% (from ~ 13% to ~ 8% in absolute terms) in response to a 6-month intervention involving a referral to a registered dietitian and an instruction to increase steps to 10,000/day (Table 3). HbA1c was also significantly reduced following the lifestyle intervention, suggesting an improvement in glycaemic control. Notably, the findings of Wong et al. [60] demonstrate the effectiveness of lifestyle interventions aimed at decreasing calorie intake and increasing energy expenditure for treating NAFLD (i.e. reducing IHTAG) in patients with and without obesity (Table 3). Although the data appears to suggest that greater relative weight loss is required in patients with obesity compared to those without obesity for remission of NAFLD; patients without obesity achieved remission of NAFLD with 3–5% weight loss whereas 7–10% weight reduction was required to achieve NAFLD remission in patients with obesity. Combined, these studies highlight the potential benefits of multidisciplinary interventions for patients with both NAFLD and T2D. However, in these studies it is unclear as to whether it is the exercise or weight-loss that is beneficial, or if the combination of changes in diet and physical activity are additive compared to either in isolation.
It is unclear whether patients with both NAFLD and T2D respond differently to an intervention compared to those with NAFLD or T2D in isolation. Konerman et al. [61] investigated the effects of a multidisciplinary lifestyle intervention in patients with the metabolic syndrome who were grouped on the basis of the presence or absence of NAFLD (diagnosed by either imaging or liver biopsy). The intervention involved one session a week for 12 or 24 weeks (participants were given the option as to which program to undertake). Each session involved a 45-min nutrition lecture focused on improving adherence to a Mediterranean diet, reducing sodium intake, portion control and other healthy eating habits. Participants also performed a 45-min supervised exercise routine during each session. The main findings were that approximately half of the patients with metabolic syndrome who were screened had NAFLD, and these patients displayed a poorer metabolic profile (higher prevalence of features of the metabolic syndrome and insulin resistance) than those with metabolic syndrome without NAFLD. However, weight loss and improvements in metabolic parameters were similar in patients with and without NAFLD after 12 and 24 weeks of the intervention (Table 3) suggesting that lifestyle interventions incorporating diet and physical activity may be equally as effective in those with both NAFLD and T2D compared to those with isolated metabolic conditions.
Multidisciplinary Interventions and NASH
Regarding more advanced NAFLD, in a randomised controlled paired liver biopsy study Promrat and colleagues [62] reported that patients who were histologically diagnosed with NASH demonstrated a reduction in the NAFLD activity score (NAS) and steatosis following an intensive 48-week lifestyle intervention (Table 3). This intervention utilised behavioural change techniques to educate and encourage participants to adopt a hypocaloric, low-fat diet and increase their physical activity levels (target of 10,000 steps a day plus 200 min a week moderate intensity exercise) [62]. Following the intervention, a large proportion of participants (67%) no longer met the histologic criteria for NASH, although degree of fibrosis appeared to be unaffected. Weight loss in this study was ~ 9.3% in the intervention group and ~ 0.2% in the control group, and the magnitude of weight loss correlated strongly with improvements in markers of NASH. The authors suggested that ~ 7% weight loss is required to improve histological markers of NASH. Further evidence that the degree of weight loss is an important mediating factor regarding improvements in NASH comes from Vilar-Gomez et al. [63], who analysed paired liver biopsies before and after a year-long lifestyle intervention where participants were encouraged to reduce their estimated energy intake by ~ 750 kcal/day and walk ~ 200 min a week (Table 3). Mean weight loss at 1 year was − 4.6 ± 3.2 kg and NASH resolution was reported in 25% of participants, with improvements in NAS (by 2 points) seen in 47% of participants. When separating participants into weight loss categories (< 5%, 5–7%, 7–10%, and > 10%) the authors observed that 7–10% weight loss was required to see histological improvements in NAFLD, and greater weight loss was associated with greater improvements, which is in line with the observations of Promrat et al. [62]. However, Vilar-Gomez et al. [63] also found that the majority (239 of 261 participants) failed to reach 7–10% weight loss, highlighting how challenging lifestyle interventions may be for this population. Why some participants failed to lose more than 7% of their body weight is unclear, but this study was performed under routine clinical care, and therefore participants received less support than was provided in other multidisciplinary intervention studies.
Impact of Patient Engagement and Support
Studies that have investigated patient performance in relation to level of support/engagement suggest a positive association between the two. A sub-study of the ‘Look AHEAD’ intervention [64] investigated the effect of an intensive lifestyle intervention which involved weekly support meetings, and monthly individual and group sessions, and compared this to a control group who only underwent three group sessions. The authors found that the intensive program led to a significant reduction in body weight and IHTAG compared to the control group (Table 3). A reduction in T2D medications was also noted in the intensive lifestyle intervention group at 12 months, and reduced IHTAG was associated with reductions in HbA1c and circulating plasma TAG, suggesting an improved overall metabolic profile. Similarly, St George et al. [65] randomised 143 patients with NAFLD to one of four intervention groups which differed in their level of support/engagement (i.e. low intensity vs. moderate intensity vs. control). The low-intensity intervention consisted of three individually tailored counselling and education sessions over a 4-week period, whereas the moderate-intensity intervention consisted of six individually tailored counselling and education sessions over a 10-week period. During the consultations a number of behaviour change techniques were delivered by nutritionists and exercise physiologists aimed at increasing physical activity to at least 150 min/week and reduce calories by ~ 400–600 kcal/day. Weight loss was greatest in the moderate-intensity group at 3 months which was associated with a greater reduction of metabolic risk factors (Table 3). Together these data demonstrate that patients who receive greater support demonstrate greater metabolic improvements in response to an intervention.
Providing increased support may increase the burden placed on resources. However, the findings of a recent study suggest that a structured web-based program may be equally as effective as a face-to-face group-based program. Mazzotti et al. [66] investigated weight loss and surrogate NAFLD indices in patients who underwent group counselling compared to those who underwent a web-based program. Both programs encouraged adherence to a Mediterranean diet and increased physical activity. The group-based program involved five 120-min weekly sessions delivered by physicians, dietitians, and a psychologist, whereas the web-based program included the same content, separated into 25–35 interactive slides. The participants were able to access the web-based content without limitations. Both groups attended the clinic for follow-up visits every 6 months. Both groups demonstrated similar weight loss at 6, 12, and 24 months, with average weight loss at 24 month of ~ 5.5% in the web-based cohort and ~ 4.2% in the group-based cohort (Table 3). Surrogate indices of NAFLD also improved to a similar extent in both groups, demonstrating that a web-based program is as effective as a group-based face-to-face intervention for patients with NAFLD and T2D. However, less than 20% of the entire cohort met the predetermined target of 10% weight loss, and normalisation of ALT was only reported in ~ 35% of the web-based cohort and ~ 20% of the group-based at 24 months. This indicates that the majority of participants undertaking either intervention failed to meet weight loss targets or reduce ALT to non-NAFLD levels and suggests that the intervention as a whole, whether web-based or group-based, needs to be optimised for this population.
Diet vs. Physical Activity Interventions
As these studies are multidisciplinary, it is challenging to decipher which aspects of the intervention are most beneficial; this is important for delivering and refining future prevention and treatment programs. When comparing diet to diet and physical activity interventions in 45 patients with T2D, Bozzetto et al. [67] reported that a high-MUFA diet reduced IHTAG, but the addition of exercise had little additive effect (Table 3). This finding suggests that diet, rather than exercise, interventions mediate improvements in markers of NAFLD in patients with T2D. Whilst this observation appears to disagree with much of the literature cited above regarding the influence of exercise on IHTAG, it is supported by the findings of Eckard et al. [68], who observed improvements in NAS following a diet, and diet and exercise intervention, but not when exercise was performed in isolation (Table 3). Conversely, in a tightly controlled inpatient study, Tamura et al. [69] randomly assigned 14 subjects to a control diet or a combined diet and exercise intervention, where the diet was matched but participants also completed 2–3 30-min sessions of walking 5–6 days/week for 2 weeks (Table 3). Both groups reduced their BMI and body fat percentage to a similar extent, and both groups showed a reduction in IHTAG. However, only the exercise group showed a reduction in intramyocellular fat (assessed by MRI/S), which was associated with improvements in clamp-derived measures of peripheral insulin sensitivity; no improvements in intramyocellular fat or peripheral insulin sensitivity were observed in the group who only altered their diet.
Taken together the evidence suggests that combined diet and aerobic exercise interventions do not appear to cause further IHTAG reductions compared with diet interventions alone when matched for weight loss. However, whilst reductions in IHTAG may be similar, a combined diet and exercise intervention may improve an individual’s overall metabolic health via alterations in other tissues (e.g. skeletal muscle) above that of diet alone. However, not all studies support this hypothesis. Otten et al. [70] investigated the effects of an ad libitum Paleolithic diet for 12 weeks in combination with 3 h a week of supervised aerobic and resistance training compared to standard care physical activity recommendations (Table 3). Weight loss was similar (~ 7 kg) in both groups, as was the reduction in peripheral insulin sensitivity during a hyperinsulinemic-euglycaemic clamp. No difference was reported in hepatic insulin sensitivity after either intervention despite IHTAG decreasing by ~ 74% in the diet group, and ~ 32% in the diet and exercise group. The smaller reduction in the latter group appears to have been driven by three participants who gained IHTAG, as removal of these individuals from analysis resulted in no significant differences between groups for IHTAG. Why these participants displayed a divergent response is unclear. Conversely, Al-Jiffri et al. [71] investigated 100 male patients with both NAFLD and T2D and randomised them to a combined diet and exercise intervention, or diet alone. Exercise consisted of treadmill running at 65–75% of heart rate max three times a week for 3 months, and the diet was a prescriptive hypocaloric diet providing 1200 kcal/day. Significant reductions in BMI (~ 4.9 kg/m2) were reported in the combined diet and exercise group, which were associated with reductions in ALT, AST, γGT, and HOMA-IR. However, no significant reductions in BMI or liver enzymes were noted in the group that had the diet intervention alone. Adherence to the respective interventions was not reported. Whilst this finding appears to be in contrast with those above, it highlights the importance of weight loss in managing NAFLD in this population.
Overall, the evidence clearly demonstrates that multidisciplinary interventions aimed at reducing body weight are beneficial for patients with both NAFLD and T2D, with greater weight loss associated with greater improvements in metabolic health. From the limited number of studies that have compared the effects of diet and physical activity it appears that it is diet that has the greatest influence on NAFLD, although increased physical activity may have additional metabolic benefits in other tissues. The level of support provided in a number of these studies is relatively high (e.g. individually tailored diet/exercise programs, regular follow-ups by diet and exercise professionals etc.). The data suggest that those who receive additional support/monitoring may respond better to an intervention. However, it is unclear as to whether upscaling interventions of this nature is feasible because of the sustained requirements of specialist staff. Further research is required regarding the ability to provide such interventions within a primary care setting and the cost implications of delivering such interventions.
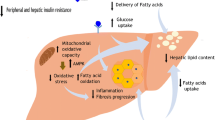
Proposed Molecular Mechanisms Underpinning Lifestyle Interventions
The mechanisms through which physical activity/exercise reduces IHTAG content have received relatively little attention. A recent hypothesis relates to the myokine interleukin-6 (IL-6) which is increased in response to exercise [72]; it is suggested that IL-6 signalling mediates exercise-induced reductions in visceral adipose tissue mass [73]. IL-6 treatment in rat hepatocytes led to increased secretion of apolipoprotein B (ApoB)-containing lipoproteins [74] and an increased secretion of TAG-rich lipoproteins may play a role in the reduction of IHTAG content [75]. Taken together, this represents a theoretical pathway through which exercise, via IL-6 signalling, may influence IHTAG. Furthermore, during sustained physical activity/exercise there is an increase in glucose uptake by skeletal muscle, which is met in part by the breakdown of glycogen in the muscle, but also from increased hepatic glucose output, mediated by the breakdown of liver glycogen and gluconeogenesis [76]. Animal evidence demonstrates that as gluconeogenesis increases so does the hydrolysis of adenosine triphosphate (ATP) resulting in an increased AMP:ATP ratio in the liver. Subsequent activation of the energy sensor AMPK and initiation of downstream signalling cascades result in a net reduction in lipogenesis and a parallel increase in fatty acid (FA) oxidation [77]. Although in this setting there is an increased provision of FA from adipose tissue to the liver for oxidation, it is plausible that over time repeated exercise may increase hepatic FA oxidation capacity. Additionally, physical activity and exercise represent a strong stimulus for adipose tissue lipolysis and FA oxidation that can persist for an extended duration (10–20 h) after the cessation of exercise, which may have implications for whole-body fat accumulation [78].
Other mechanisms through which physical activity/exercise may mediate improvements in NAFLD may relate to insulin sensitising in skeletal muscle. Increased physical activity/exercise is known to improve whole-body insulin sensitivity [79, 80], which appears to be attributable to alterations in skeletal muscle insulin sensitivity [81, 82]. This may reduce postprandial insulin excursions which would have implications for hepatic FA metabolism; elevated insulin concentrations have been shown to suppress VLDL-1 ApoB production, stimulate activity of lipogenic enzymes, and upregulate de novo lipogenesis (DNL) [83], which may increase the production and retention of IHTAG. Furthermore, an intermediate in the DNL pathway, malonyl-CoA, inhibits carnitine palmitoyltransferase 1 activity, partitioning FA away from oxidation and towards esterification pathways [83, 84]. However, at present these mechanisms are speculative and the influence of exercise on intrahepatic FA partitioning in humans requires further investigation.
There is also limited available evidence regarding the mechanisms underpinning the effects of dietary interventions on IHTAG. It is plausible that during hypocaloric diets, the reduction in energy intake reduces the availability of substrates for the production of IHTAG (e.g. FA for esterification and non-lipid precursors for DNL). Support for this comes from animal studies where a downregulation in the expression of genes involved in hepatic lipogenesis has been demonstrated after hypocaloric feeding [85]. Human data also demonstrates reductions in the lipogenic index (used as a proxy for DNL) in response to weight loss, which was associated with reduced IHTAG [86], although we have previously shown that plasma indices of DNL are not representative of isotopically determined DNL [87]. Improvements in whole-body and hepatic insulin sensitivity have also been observed in response to hypocaloric diets [48, 50, 52, 88]. Improvements in insulin sensitivity have previously been associated with reductions in adipocyte size rather than reductions in body weight per se [88, 89]. It has been proposed that reductions in cell volume may alleviate cellular hypoxia which has been associated with endoplasmic reticulum stress and inflammation [90]. However, further hepatocentric investigations regarding the effect of hypocaloric diets and weight loss are required in order to elucidate the molecular mechanisms. It is reasonable to assume that in those undergoing multidisciplinary interventions, where both hypocaloric diet and exercise interventions are employed, there are multiple simultaneous mechanisms at play mediating metabolic improvements.
Conclusions
From the available evidence it is clear that increasing physical activity/exercise is effective in improving metabolic parameters in patients with both NAFLD and T2D. Specifically, it appears that the addition of any kind of exercise (i.e. resistance, aerobic, or high-intensity intermittent exercise) is beneficial for patients with both NAFLD and T2D. These effects appear to occur independently of changes in body weight. Hypocaloric diets leading to weight loss are also effective in improving metabolic parameters pertinent to NAFLD and T2D. Where data is available it appears that 7–10% weight loss is required in order to observe beneficial effects on NAFLD parameters, although this appears to be dependent on the population being studied as smaller amounts of weight loss (3–5%) appear to be beneficial in patients with NAFLD without obesity. It remains unclear whether there is an optimal macronutrient composition for hypocaloric diets in regards to treating patients with both NAFLD and T2D. Combining diet and exercise interventions also appears to be an effective treatment strategy in patients with NAFLD and T2D. However, it is unclear if multidisciplinary interventions are more effective than diet and exercise interventions in isolation in this population. Comparing treatment strategies in patients with both NAFLD and T2D is necessary in order to develop future cost-effective treatment strategies.
Whilst lifestyle interventions are effective in a large proportion of individuals, there exists a subset who demonstrate no clinical improvements relevant to NAFLD in response to lifestyle interventions. It is plausible that more intensive treatments are necessary in these individuals. For example, Moolla et al. [91] recently demonstrated the effectiveness of a multidisciplinary hepatology clinic that combines lifestyle intervention with pharmacological treatment in improving liver-related and cardiometabolic health in patients with both NAFLD and poorly controlled T2D. Health economic modelling also suggests that this intervention was cost effective in treating this population. Other, more extreme interventions such as bariatric surgery may also be necessary in certain populations.
Future Directions
There are a number of open questions regarding lifestyle interventions in patients with both NAFLD and T2D. Firstly, despite the strong association between NAFLD and T2D there exists a relatively small number of intervention studies which have recruited patients with both NAFLD and T2D, and some studies actively exclude those with coexisting NAFLD and T2D. It is unclear if findings from patients with NAFLD without T2D can be directly extrapolated to patients with both NAFLD and T2D, or if certain aspects of an individual’s physiology that led to the progression of these coexisting diseases would influence disease remission or response to an intervention. Future research should aim to establish whether patients with both NAFLD and T2D respond to interventions in a similar manner to those with NAFLD or T2D in isolation. Elucidating the mechanisms underpinning lifestyle interventions would also aid in the development of future prevention and treatment strategies.
Moreover, the longest studies to date are 12 months in duration, and it is unclear whether or not participants were able to continue implementing changes once outside the research setting or in the case of hypocaloric diets whether longer-term interventions are safe and feasible. Questions also remain as to the optimal macronutrient composition of hypocaloric diets. A further area of research relates to the scalability of these interventions, as many studies required a large number of specialist staff for extended periods of time. Analysing the cost effectiveness of such interventions is vital in deciding upon future treatment strategies for patients with both NAFLD and T2D.
References
Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–23.
Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5(3):211–8.
Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387–95.
Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286–92.
McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–9.
European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402.
Dyson JK, McPherson S, Anstee QM. Non-alcoholic fatty liver disease: non-invasive investigation and risk stratification. J Clin Pathol. 2013;66(12):1033–45.
Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128(7):1898–906.
Ballestri S, Nascimbeni F, Lugari S, Lonardo A, Francica G. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert Rev Gastroenterol Hepatol. 2019;13(7):667–81.
Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–50.
van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and point-resolved 1H MR spectroscopy. Radiology. 2010;256(1):159–68.
Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288(2):E462–8.
Dumas ME, Kinross J, Nicholson JK. Metabolic phenotyping and systems biology approaches to understanding metabolic syndrome and fatty liver disease. Gastroenterology. 2014;146(1):46–62.
Luukkonen PK, Zhou Y, Hyotylainen T, et al. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J Hepatol. 2016;65(6):1263–5.
Nascimbeni F, Pais R, Bellentani S, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59(4):859–71.
Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419–30.
Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13(4):511–31.
Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132(2):112–7.
Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–89.
James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29(3):495–501.
Nomura H, Kashiwagi S, Hayashi J, et al. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27(2):142–9.
Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(1):27–38.
Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–10.
Dai W, Ye L, Liu A, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltimore). 2017;96(39):e8179.
Ballestri S, Zona S, Targher G, et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016;31(5):936–44.
Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41(2):372–82.
Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122–30.
Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65(8):1096–108.
Ryysy L, Hakkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49(5):749–58.
Kim KS, Lee BW, Kim YJ, et al. Nonalcoholic fatty liver disease and diabetes: part II: treatment. Diabetes Metab J. 2019;43(2):127–43.
Koutoukidis DA, Astbury NM, Tudor KE, et al. Association of weight loss interventions with changes in biomarkers of nonalcoholic fatty liver disease: a systematic review and meta-analysis. JAMA Intern Med. 2019. https://doi.org/10.1001/jamainternmed.2019.2248.
Hallsworth K, Thoma C, Moore S, et al. Non-alcoholic fatty liver disease is associated with higher levels of objectively measured sedentary behaviour and lower levels of physical activity than matched healthy controls. Frontline Gastroenterol. 2015;6(1):44–51.
Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529–42.
Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527–45.
Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57(1):157–66.
Sargeant JA, Gray LJ, Bodicoat DH, et al. The effect of exercise training on intrahepatic triglyceride and hepatic insulin sensitivity: a systematic review and meta-analysis. Obes Rev. 2018;19(10):1446–59.
Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60(9):1278–83.
Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology. 2013;58(4):1287–95.
Stutts WC. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. 2002;50(11):499–507.
Bartlett JD, Close GL, MacLaren DP, et al. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. J Sports Sci. 2011;29(6):547–53.
Thum JS, Parsons G, Whittle T, Astorino TA. High-intensity interval training elicits higher enjoyment than moderate intensity continuous exercise. PLoS One. 2017;12(1):e0166299.
Cassidy S, Thoma C, Hallsworth K, et al. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2016;59(1):56–66.
Houghton D, Thoma C, Hallsworth K, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96–102.e3.
Hallsworth K, Thoma C, Hollingsworth KG, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond). 2015;129(12):1097–105.
Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine (Baltimore). 2019;98(12):e14918.
Keating SE, Hackett DA, Parker HM, et al. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174–82.
Huber Y, Pfirrmann D, Gebhardt I, et al. Improvement of non-invasive markers of NAFLD from an individualised, web-based exercise program. Aliment Pharmacol Ther. 2019;50(8):930–9.
Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54(10):2506–14.
Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391(10120):541–51.
Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for beta cell recovery. Cell Metab. 2018;28(4):547–556.e3.
Steven S, Hollingsworth KG, Al-Mrabeh A, et al. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care. 2016;39(5):808–15.
Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603–8.
Vilar-Gomez E, Athinarayanan SJ, Adams RN, et al. Post hoc analyses of surrogate markers of non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in patients with type 2 diabetes in a digitally supported continuous care intervention: an open-label, non-randomised controlled study. BMJ Open. 2019;9(2):e023597.
Parker HM, Johnson NA, Burdon CA, et al. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;56(4):944–51.
Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49(2):137–44.
De Feo P, Fatone C, Burani P, et al. An innovative model for changing the lifestyles of persons with obesity and/or type 2 diabetes mellitus. J Endocrinol Investig. 2011;34(10):e349–54.
Reginato E, Pippi R, Aiello C, et al. Effect of short term intensive lifestyle intervention on hepatic steatosis indexes in adults with obesity and/or type 2 diabetes. J Clin Med. 2019. https://doi.org/10.3390/jcm8060851.
Sun WH, Song MQ, Jiang CQ, et al. Lifestyle intervention in non-alcoholic fatty liver disease in Chengyang District, Qingdao, China. World J Hepatol. 2012;4(7):224–30.
Thomas EL, Brynes AE, Hamilton G, et al. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12(36):5813–9.
Wong VW, Wong GL, Chan RS, et al. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol. 2018;69(6):1349–56.
Konerman MA, Walden P, Joseph M, et al. Impact of a structured lifestyle programme on patients with metabolic syndrome complicated by non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49(3):296–307.
Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–9.
Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378.e5 (quiz e14–5).
Lazo M, Solga SF, Horska A, et al. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33(10):2156–63.
St George A, Bauman A, Johnston A, et al. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J Gastroenterol Hepatol. 2009;24(3):399–407.
Mazzotti A, Caletti MT, Brodosi L, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69(5):1155–63.
Bozzetto L, Prinster A, Annuzzi G, et al. Liver fat is reduced by an isoenergetic MUFA diet in a controlled randomized study in type 2 diabetic patients. Diabetes Care. 2012;35(7):1429–35.
Eckard C, Cole R, Lockwood J, et al. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: a randomized trial. Therap Adv Gastroenterol. 2013;6(4):249–59.
Tamura Y, Tanaka Y, Sato F, et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90(6):3191–6.
Otten J, Stomby A, Waling M, et al. A heterogeneous response of liver and skeletal muscle fat to the combination of a Paleolithic diet and exercise in obese individuals with type 2 diabetes: a randomised controlled trial. Diabetologia. 2018;61(7):1548–59.
Al-Jiffri O, Al-Sharif FM, Abd El-Kader SM, Ashmawy EM. Weight reduction improves markers of hepatic function and insulin resistance in type-2 diabetic patients with non-alcoholic fatty liver. Afr Health Sci. 2013;13(3):667–72.
Ellingsgaard H, Hojman P, Pedersen BK. Exercise and health—emerging roles of IL-6. Curr Opin Physiol. 2019;10:49–54.
Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2019;29(4):844–855.e3.
Sparks JD, Cianci J, Jokinen J, Chen LS, Sparks CE. Interleukin-6 mediates hepatic hypersecretion of apolipoprotein B. Am J Physiol Gastrointest Liver Physiol. 2010;299(4):G980–9.
Hodson L, Frayn KN. Hepatic fatty acid partitioning. Curr Opin Lipidol. 2011;22(3):216–24.
Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296(1):E11–21.
Trefts E, Williams AS, Wasserman DH. Exercise and the regulation of hepatic metabolism. Prog Mol Biol Transl. 2015;135:203–25.
Thompson D, Karpe F, Lafontan M, Frayn K. Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev. 2012;92(1):157–91.
Mann S, Beedie C, Balducci S, et al. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab Res Rev. 2014;30(4):257–68.
Way KL, Hackett DA, Baker MK, Johnson NA. The effect of regular exercise on insulin sensitivity in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab J. 2016;40(4):253–71.
Malin SK, Haus JM, Solomon TP, et al. Insulin sensitivity and metabolic flexibility following exercise training among different obese insulin-resistant phenotypes. Am J Physiol Endocrinol Metab. 2013;305(10):E1292–8.
Reichkendler MH, Auerbach P, Rosenkilde M, et al. Exercise training favors increased insulin-stimulated glucose uptake in skeletal muscle in contrast to adipose tissue: a randomized study using FDG PET imaging. Am J Physiol Endocrinol Metab. 2013;305(4):E496–506.
Hodson L, Fielding BA. Trafficking and partitioning of fatty acids: the transition from fasted to fed state. Clin Lipidol. 2010;5(1):131–44.
Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23(2):201–29.
Margolis LM, Rivas DA, Ezzyat Y, et al. Calorie restricted high protein diets downregulate lipogenesis and lower intrahepatic triglyceride concentrations in male rats. Nutrients. 2016. https://doi.org/10.3390/nu8090571.
Sevastianova K, Santos A, Kotronen A, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96(4):727–34.
Rosqvist F, McNeil CA, Pramfalk C, et al. Fasting hepatic de novo lipogenesis is not reliably assessed using circulating fatty acid markers. Am J Clin Nutr. 2019;109(2):260–8.
McLaughlin T, Abbasi F, Lamendola C, et al. Dietary weight loss in insulin-resistant non-obese humans: metabolic benefits and relationship to adipose cell size. Nutr Metab Cardiovasc Dis. 2019;29(1):62–8.
Andersson DP, Eriksson Hogling D, Thorell A, et al. Changes in subcutaneous fat cell volume and insulin sensitivity after weight loss. Diabetes Care. 2014;37(7):1831–6.
Kloting N, Bluher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15(4):277–87.
Moolla A, Motohashi K, Marjot T, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Frontline Gastroenterol. 2019;10(4):337–46.
Bacchi E, Negri C, Zanolin ME, et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: a randomized controlled trial (the RAED2 study). Diabetes Care. 2012;35(4):676–82.
Acknowledgements
Funding
No Rapid Service Fee or Open Access charge was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Leanne Hodson is a British Heart Foundation (BHF) Senior Research Fellow in Basic Science but has nothing else to disclose. Siôn A. Parry has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11890023.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Parry, S.A., Hodson, L. Managing NAFLD in Type 2 Diabetes: The Effect of Lifestyle Interventions, a Narrative Review. Adv Ther 37, 1381–1406 (2020). https://doi.org/10.1007/s12325-020-01281-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01281-6