Abstract
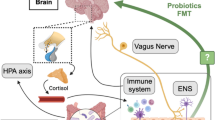
The human gut microbiome partakes in a bidirectional communication pathway with the central nervous system (CNS), named the microbiota–gut–brain axis. The microbiota–gut–brain axis is believed to modulate various central processes through the vagus nerve as well as production of microbial metabolites and immune mediators which trigger changes in neurotransmission, neuroinflammation, and behavior. Little is understood about the utilization of microbiome manipulation to treat disease. Though studies exploring the role of the microbiome in various disease processes have shown promise, mechanisms remain unclear and evidence-based treatments for most illnesses have not yet been developed. The animal studies reviewed here offer an excellent array of basic science research that continues to clarify mechanisms by which the microbiome may affect mental health. More evidence is needed, particularly as it relates to translating this work to human subjects. The studies presented in this paper largely demonstrate encouraging results in the treatment of depression. Limitations include small sample sizes and heterogeneous methodology. The exact mechanism by which the gut microbiota causes or alters neuropsychiatric disease states is not fully understood. In this review, we focus on recent studies investigating the relationship between gut microbiome dysbiosis and the pathogenesis of depression. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The microbiota–gut–brain axis is believed to modulate various central processes through the vagus nerve as well as production of microbial metabolites and immune mediators which trigger changes in neurotransmission, neuroinflammation, and behavior. |
Little is understood about the utilization of microbiome manipulation to treat disease. |
Though studies exploring the role of the microbiome in various disease processes have shown promise, mechanisms remain unclear and evidence-based treatments for most illnesses have not yet been developed. |
The animal studies reviewed here offer an excellent array of basic science research that continues to clarify mechanisms by which the microbiome may affect mental health. |
In this review, we focus on recent studies investigating the relationship between gut microbiome dysbiosis and the pathogenesis of depression. |
Introduction
The human gut microbiome, comprised of approximately 1800 different phyla and 40,000 bacterial species, has been implicated in numerous aspects of human health and disease [1]. It partakes in a bidirectional communication pathway with the central nervous system (CNS), aptly named the microbiota–gut–brain axis. The microbiota–gut–brain axis is believed to modulate various central processes through the vagus nerve as well as production of microbial metabolites and immune mediators which trigger changes in neurotransmission, neuroinflammation, and behavior [2–5]. Disruptions to the gut microbiome have been correlated with several neuropsychiatric disorders, including Parkinson’s disease, autism, schizophrenia, and depression [6–9]. The exact mechanism by which the gut microbiota causes or alters neuropsychiatric disease states is not fully understood. Further studies are required to elucidate the role of the microbiota–gut–brain axis, with the goal of preventing disease, identifying new therapeutic targets, and improving treatments. In this review, we focus on recent studies investigating the relationship between gut microbiome dysbiosis and the pathogenesis of depression.
Epidemiology
Depression is one of the most prevalent mental health disorders in the USA and the second leading cause of disability worldwide [10]. A major depressive episode is defined as a depressed mood and/or loss of interest or pleasure in life activities for at least 2 weeks, with at least five symptoms that disrupt social interactions, work, or other important areas of daily life [11]. This may include symptoms such as unintentional weight change, insomnia or hypersomnia, agitation or psychomotor retardation, fatigue, or feelings of worthlessness or guilt. In 2017, 17.3 million adults (6.8%) and 3.2 million adolescents (13.3%) in the USA experienced at least one major depressive episode [12].
In addition to causing significant functional impairment, depression is also associated with substantial economic burden. From 2005 to 2010, the economic burden of individuals with major depressive disorders (MDD) in adults increased from $173.2 to $210.5 billion [13]. Medical and pharmaceutical services directly related to the treatment of MDD accounted for $27.7 billion of the $210.5 billion total cost in 2010. The remaining costs were primarily those associated with comorbidities incurred by persons with MDD, though suicide-related and workplace costs also contributed to the total economic burden. In light of these estimates, it evident that depression is a complex disorder that greatly impacts both individuals and society. Implementation of preventative measures and effective interventions are required in order to address the challenges that depression presents.
Risk Factors for Dysbiosis
Numerous risk factors have been proposed in the pathogenesis of gut dysbiosis. The use of antibiotics has been well documented, resulting in both short-term and long-term alterations in the composition of the gut microbiome [14–17]. Reproducible gut microbiome alterations have also been demonstrated with obesity as well as high-fat and high-sugar diets [18–21]. Environmental factors at various stages of life are also believed to influence the development of gut dysbiosis. Changes in microbiome diversity at infancy have been linked to the mode of delivery, feeding type, and hospital environment [22, 23]. Exposure to xenobiotics, such as heavy metals and pesticides, as well as social stressors is also associated with gut dysbiosis [24, 25]. In addition to environmental factors, twin studies have revealed a genetic component in the development of the gut microbiome.
Animal Studies
Gut Microbiota and Depression
Kelly et al. investigated alterations in depression-associated gut microbiota composition in humans and further examined its effects on neurobehavior in rats [26]. Fecal, salivary, and plasma samples were collected from 34 patients with depression and 33 healthy patients in order to assess microbiota composition, hypothalamic–pituitary–adrenal (HPA) axis function, immune activation, tryptophan metabolism, functional consequences, and gut permeability. Fecal samples from the three most severely depressed male patients were pooled and transplanted into 13 adult male rats that were previously treated with antibiotics. Behavior, microbiota composition, HPA axis function, immune activation, tryptophan metabolism, intestinal transit time, functional consequences, and gut permeability were assessed and compared to samples from 15 adult male rats in the control group.
In the patient studies, the authors found significantly increased levels of interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor alpha (TNFα), and C-reactive protein (CRP) (p < 0.02) as well as a higher kynurenine/tryptophan ratio (p = 0.049) in patients within the depressed group. Total cortisol output was also increased (p = 0.05), although there was neither a difference in the delta cortisol response nor baseline cortisol levels upon awakening across both groups. Fecal samples from the depressed group showed decreased microbiota richness (p = 0.005), total observed species (p = 0.002), and phylogenetic diversity (p = 0.001). The most pronounced difference was observed in the reduction of the Prevotellaceae family and Prevotella genus. There were no significant differences in plasma lipopolysaccharide binding protein levels or short chain fatty acids, though depressive symptoms negatively correlated with daily fiber intake.
Rats in the depressed group exhibited anhedonia-like and anxiety-like behaviors when compared to the control group. Plasma kynurenine levels (p = 0.029) and the kynurenine/tryptophan ratio (p = 0.008) were significantly increased in the depressed group, and plasma CRP levels revealed an upward trend, though there were no significant differences across various cytokine or corticosterone levels. Rats in the depressed group also demonstrated a significant increase in intestinal transit time (p = 0.013). Fecal samples in the depressed group revealed decreased microbiota richness (p = 0.004), observed species (p = 0.006), and phylogenetic diversity (p = 0.006). There were no significant differences in lipopolysaccharide binding protein levels or short chain fatty acids.
By transplanting fecal matter from patients with depression to microbiota-depleted rats, the authors were able to induce certain behavioral and physiological features of the depressive phenotype, specifically those pertaining to gut microbiota richness and diversity, tryptophan metabolism, and immune function. From these findings, the authors concluded that alterations in the gut microbiota may play a causal role in the pathogenesis of depression. A potential confounder is that many patients in the depressed group were prescribed antidepressant medications, indicative of a potential serotonin-driven alteration in the gut microbiota. Further studies will be necessary to fully elucidate the role of gut microbiota in the development of a depressive phenotype, especially when examining the impact of gut dysbiosis on neuroendocrine and neuroimmune signaling pathways in the gut–brain–microbiota axis.
Gut Microbiota Depletion in Adult Rats
A notable finding in microbiome research is that greater species diversity among bacteria colonizing the gut appears protective against various ailments. To assess the effects of disrupting a presumably healthy microbiome, Hoban et al. investigated the behavioral and neurochemical consequences of chronic gut microbiota depletion during adulthood within rats [27]. A 6-week course of antibiotics was administered in drinking water to ten adult male rats in order to deplete intestinal microbiota, while ten adult male rats in the control group received autoclaved water devoid of any antibiotics. After the 6 weeks, all rats underwent testing to assess anxiety-like behaviors, depressive-like behaviors, spatial learning, novel object recognition, somatic pain sensitivity, colorectal distention, brain monoamine levels, corticosterone levels, microbiota composition, and gene expression in the CNS.
The authors found that antibiotic treatment resulted in significant depressive-like behaviors (p < 0.04), but demonstrated no impact on anxiety-like behaviors. Chronic antibiotic treatment was also associated with impaired spatial learning (p = 0.037) and lower visceral hypersensitivity (p = 0.015). Considering monoamines, rats treated with antibiotics exhibited a reduction in 5-hydroxytryptamine (5-HT) and an increase in 5-hydroxyindoleacetic acid (5-HIAA)/5-HT turnover in the hippocampus (p = 0.0004). Tryptophan levels also increased in antibiotic-treated rats (p = 0.032). Additionally, an increase in noradrenaline in the striatum (p < 0.003) and increases in levodopa (l-DOPA) in the prefrontal cortex and hippocampus (p < 0.0001) were noted. Dopamine precursor homovanillic acid (HVA) levels were also increased in the prefrontal cortex and hippocampus (p < 0.05). No significant difference was noted in plasma corticosterone levels in control versus antibiotic-treated rats. Analysis of gene expression showed decreased levels of glucocorticoid receptor Nr3c1 (p < 0.05) and corticotrophin-releasing hormone receptor 1 (p < 0.01) in the hippocampus and amygdala of antibiotic-treated rats, while Bdnf levels were increased in the amygdala (p < 0.01). Decreased levels of Crch1 were also noted in the hippocampus and amygdala (p < 0.01). Lastly, rats treated with antibiotics exhibited altered microbial diversity, with a significant decrease in Firmicutes and Bacteroidetes and an increase in Proteobacteria and Cyanobacteria.
From these findings, the authors were able to identify a distinct phenotype, which included depressive-like behaviors and impaired cognition, that was associated with antibiotic-induced microbiota depletion in rats during adulthood. Furthermore, the study corroborated existing literature on the importance of the gut microbiota on tryptophan availability and the CNS serotonergic system. Chronic antibiotic exposure decreased the diversity and richness of the gut microbiota, coinciding with the display of depressive-like behavior. Decreased levels of hippocampal 5-HT and 5-HT/5HIAA turnover and altered levels of l-DOPA and HVA reflected a dysregulation of monoamine synthesis and degradation, indicating that dysbiosis may profoundly impact neurotransmitter systems.
Adolescent Stress-Induced Cognitive and Microbiome Changes by Diet
Provensi et al. investigated the preventative effects of a diet enriched with ω-3 polyunsaturated fatty acids (ω-3 PUFAs) and vitamin A on stress-induced cognitive behavior and the gut microbiome [28]. Male rats were assigned to three experimental groups: non-stressed rats with the control diet (NSCD), rats subjected to the social instability protocol with the control diet (SCD), and rats subjected to the social instability protocol with the ω-3 PUFA/vitamin A-enriched diet (SED). Rats subjected to social instability underwent various changes in their housing conditions for 15 days, while non-stressed rats were left undisturbed. Following this period, rats from all three experimental groups were monitored from adolescence to adulthood with behavioral, neurochemical, and intestinal microbiota assessments.
The authors found that SCD rats gained and maintained less weight from adolescence through adulthood when compared to NSCD rats (p < 0.01). This effect was counteracted by the ω-3 PUFA/vitamin A-enriched diet (p < 0.01). Regarding long-term memory, adolescent SCD rats were unable to discriminate between two objects, an effect that persisted into adulthood. The enriched diet prevented this effect, as SED rats displayed no stress-induced impairment of object discrimination in adolescence or adulthood (p < 0.001). Similarly, SCD rats also experienced long-lasting impairments in contextual fear memory when compared to SED rats (p < 0.001). Locomotor activity, anhedonia-like behavior, and anxiety-related behavior were comparable across all three cohorts.
Considering brain plasticity, expression of brain-derived neurotrophic factor (BDNF) was decreased in the hippocampus of adolescent and adults SCD rats compared to the NSCD and SED rats (p < 0.01). Increased levels of BDNF were also found in the frontal cortex of adolescent and adult rats SED rats (p < 0.05). In the hippocampus and frontal cortex of SED rats, a significant increase in synaptophysin expression was noted as well (p < 0.05). Analysis of the gut microbiota of SED adolescent rats demonstrated increased alpha diversity when compared to both SCD and NSCD rats (p < 0.05), though this effect did not persist through adulthood. There was a shift in the microbiome composition and beta diversity in SCD adolescent rats when compared to NSCD adolescent rats (p < 0.05). Analysis of gut microbiota revealed a decreased relative abundance of the Lachnospiraceae and Ruminococcaceae families and an increased relative abundance of the Eubacterium genus and Coriobacteriaceae family. This shift was almost entirely prevented when stressed adolescent rats were fed the enriched diet (p < 0.1) and persisted throughout adulthood. Furthermore, social instability stress reduced the concentration of short chain fatty acids (p < 0.05) in SCD adolescent rats. In contrast, SED adolescent rats showed an overall increase in branched short chain fatty acids (p < 0.001).
In examining the effects of the ω-3 PUFA/vitamin A-enriched diet in adolescent and adult rats, the authors stressed the fundamental role of nutrition and dietary intervention on neurobehavioral development and the gut microbiome. The administration of the ω-3 PUFA/vitamin A-enriched diet prevented memory and BDNF decline associated with social instability stress induced during adolescence in rats. BDNF in the hippocampus plays a significant role in the initiation of fear memory consolidation, an ability that stressed rats fed a controlled diet lacked.
Furthermore, stressed rats exhibited changes to their gut microbiome composition. Adolescent stress resulted in decreased Lachnospiraceae and Ruminococcaceae, which have also been noted to be decreased in patients with depressive disorders [29]. Additionally, increases in Coriobacteriaceae and Eubacteriaceae are associated with experimental colitis in rats [30]. Though these negative changes did not persist into adulthood, administration of an ω-3 PUFA/vitamin A-enriched diet prevented these modifications during adolescence and furthermore resulted in a long-lasting shift in beta diversity. The enriched diet also increased the production of branched short chain fatty acids, which are strongly correlated with improved anxiety-like and depressive-like behaviors. While these results imply that optimization of diet may play a pivotal role in the amelioration of stress-related behaviors, further studies are necessary to determine whether a causal relationship exists between an ω-3 PUFA/vitamin A-enriched diet and the gut microbiome and behavior.
Gut Microbiota Depletion from Early Adolescence
Desbonnet et al. examined the effects of gut microbiota depletion from early adolescence in mice on brain development and behavior [31]. At postnatal day 21, adolescent mice were assigned to one of two groups: the control group (n = 14) which received autoclaved drinking water or the treatment group (n = 15) which received an antibiotic cocktail in drinking water. Starting at postnatal day 55, mice were assessed for behavior, corticosterone response to acute stress, gut microbiota composition, BDNF and hypothalamus neuropeptide expression levels, brain monoamines, and tryptophan metabolism, as well as postmortem weights of the spleen and adrenal glands.
A reduction in postmortem spleen/body weight ratio was observed in antibiotic-treated mice when compared to control mice (p < 0.0005), though no significant difference was noted in postmortem adrenal gland/body weight ratio. Behavioral testing revealed a diminished ability to recognize novel objects, a reduced preference for cued food, and increased fecal excretion in antibiotic-treated mice when compared to control mice (p < 0.05). Antibiotic-treated mice also spent significantly more time in the light chamber in the light/box dark test (p < 0.05). While baseline corticosterone levels between control and antibiotic-treated mice did not differ, the experience of acute restraint stress induced an increase in corticosterone levels in antibiotic-treated mice (p < 0.05).
Regarding gut microbiota composition, antibiotic treatment reduced the number of observed bacteria species, phylogenic diversity, and species richness (p < 0.0001). At the phylum level, there was a decrease in the relative abundances of Firmicutes (p < 0.005) and Bacteroides (p < 0.0001), while an increase in the relative abundances of Proteobacteria and Cyanobacteria was noted (p < 0.0001). At the family level, reductions in the relative abundances of Prevotellaceae (p < 0.0001), Rikenellaceae (p < 0.0001), and Incertae Sedis XI (p < 0.0005) were noted. Additionally, acute stress further altered the gut microbiota in both control and antibiotic-treated mice. While control mice exhibited an increase in the number of observed bacterial species and phylogenetic diversity, antibiotic-treated mice had a reduction in the number of observed bacterial species, phylogenetic diversity, and species richness (p < 0.02). In particular, acute stress increased Rikenellaceae in control mice (p < 0.05), while no change was observed in antibiotic-treatment mice.
Antibiotic treatment increased tryptophan levels (p < 0.0005) and decreased kynurenine levels (p < 0.0001) relative to controls. Antibiotic treatment also significantly reduced hippocampal BDNF (p < 0.05) as well as hypothalamic oxytocin (p < 0.02) and vasopressin (p < 0.0001) expression levels. Furthermore, increased levels of brain monoamines noradrenaline and 5-HIAA in the hippocampus (p < 0.0001) were noted in in antibiotic-treated mice, though the rise in 5-HIAA was only seen in non-stressed antibiotic-treated mice. Increases in l-DOPA (p < 0.0001) and HVA (p < 0.005) in the amygdala were also noted in antibiotic-treated mice.
Additionally, acute stress also appeared to impact tryptophan metabolism and monoamine concentrations. A decrease in tryptophan levels (p < 0.0005) and an increase in the kynurenine/tryptophan ratio (p < 0.01) were seen in both control and antibiotic-treated mice. While no difference was observed in hippocampal BDNF or hypothalamus neuropeptides between the two groups, acute stress induced elevations in 5-HIAA (p < 0.0001) and the 5-HIAA/serotonin ratio (p < 0.002) in the hippocampus, as well as in the prefrontal cortex (p < 0.005). Furthermore, acute stress increased HVA levels in the amygdala, particularly in the antibiotic-treated group (p < 0.05).
In light of these findings, the authors concluded that bacterial depletion and gut microbiota restructuring from early adolescence may significantly alter brain development and behavior. Gut dysbiosis following antibiotic treatment was accompanied by changes in concentrations of neuromodulatory substances and neuropeptides, and levels of growth factor BDNF. This also correlated with altered cognition and behavior, including a reduced capacity to recognize novel objects, reduced anxiety, non-spatial memory deficits, and impaired performance in the social transmission of food preference test. While the data suggests that the disruption of the gut microbiota during adolescence may have profound impacts on the microbiota–gut–brain axis and subsequently cognition and behavior, future studies should elucidate the role of bacterial depletion in more defined periods of brain development, as well as the mechanisms by which gut microbes modulate the levels and activity of neuromodulatory substances, neuropeptides, and growth factors.
Bifidobacterium and 5-Hydroxytryptophan Regulation
Tian et al. investigated the effects of Bifidobacterium administration on 5-hydroxytryptophan (5-HTP) synthesis regulation, depressive behaviors, and gut microbiome composition [32]. Adult mice were randomly assigned by body weight to either control or experimental groups, consisting of the depression group, fluoxetine group (positive control), and probiotic group. The experimental groups underwent the chronic unpredictable mild stress protocol for 5 weeks, after which all mice underwent behavioral, neurobiological, immunological, and fecal testing. Additionally, RIN14B cells were used as a putative enterochromaffin cell model to assess the impact of the E41 and M2CF22M7 strains of Bifidobacterium on 5-HTP synthesis.
Regarding depressive behaviors, administration of the strains E41, M2CF22M7, F45BB, GM59, and S60 significantly increased the swimming time in the probiotic group when compared to the depression group (p < 0.05). Administration of E41, M2CF22M7, C9, H28L1, HH160497, and MSPC591 also significantly reversed the stress-induced anhedonia in the probiotic group (p < 0.05). The depression group exhibited a decrease in the cecum short chain fatty acid (SCFA) butyrate (p < 0.001), an effect that was reversed by administration of E41, F45BBm, and S60 in the probiotic group (p < 0.05). Increased butyrate levels positively correlated with improved performance in the open field test, which was used to assess anxiety-like behavior.
Regarding neurobiological and immunological testing, administration of the strains E41, S60, and H28L1 reversed the deficits of hippocampal 5-HT and 5-HTP observed in the depression group (p < 0.05). Administration of E41, M2CF22M7, and F45BB also increased BDNF levels in the prefrontal cortex (p < 0.05). Analysis of RIN14B cells revealed significant increases in Tph1 mRNA (p < 0.0001) and 5-HTP (p < 0.01) with administration of E41 and M2CF22M7. Additionally, a decrease in serum corticosterone correlated with administration of M2CF22M7, S60, and H28L1 (p < 0.05). The strain F45BB was also associated with a signification reduction in Treg cells (p < 0.01).
Assessment of microbiota dysbiosis revealed a dramatic alteration of the gut microbial structure when comparing mice under chronic stress to the control group mice, with a decrease in Bacteroidetes and an increase in Actinobacteria observed in the depression group (p < 0.05). The relative abundances of Rikenellaceae and Lachnospiraceae were also decreased, while the relative abundances of Veillonellaceae, Desulfovibrio, and Lactobacillus were increased. Treatment with E41 and M2CF22M7 significantly reduced the abundance of Veillonellaceae, and treatment with E41 additionally decreased the abundance of Desulfovibrio. Overall, administration of probiotics increased alpha diversity of the microbiome as measured by the Chao 1 index (p < 0.01). Furthermore, assessment of the functional pathways of the microbiota revealed 20 different functional categories affecting metabolism and gene information processing between the control and depression groups. Most notably, Bifidobacterium treatment resulted in significant upregulation of glutamatergic synapses and phenylalanine/tyrosine/tryptophan biosynthesis (p < 0.05).
In light of these findings, the authors concluded that administration of certain strains of Bifidobacterium may exert antidepressive effects through the regulation of gut 5-HTP synthesis. In this study, mice in the depression group exhibited deficits of hippocampal 5-HT and 5-HTP and prefrontal cortex BDNF. Additionally, in human studies, patients with depression have exhibited elevated cortisol levels as a result of long-term stress and sustained activation of the HPA, which further exacerbates deficiencies in 5-HT and BDNF [33, 34].
Treatment with Bifidobacterium was able to not only reverse the deficiencies in 5-HT and BDNF but also decrease serum corticosterone levels. Furthermore, administration of E41 and M2CF22M7 to RIN14B cells enhanced 5-HTP synthesis without affecting the final production of 5-HT. Additionally, probiotic treatment upregulated tryptophan biosynthesis and glutamatergic synapses. Glutamate is the main excitatory neurotransmitter in the CNS and has previously been reported to display antidepressant effects in ketamine use [35]. Consequently, the mice treated with probiotics in this study displayed improved behavioral and neurological performance when compared to the mice in the depression group. While these findings suggest that probiotics may improve the synaptic signaling pathway and neuronal connections that are implicated in the pathophysiology of depression, further studies are necessary to fully understand the precise mechanism by which probiotics can modulate the gut microbiome and the gut–brain–axis.
Fructo-Oligosaccharides, Galacto-Oligosaccharides, and Chronic Stress
Burokas et al. examined the effects of prebiotic fructo-oligosaccharides (FOS) and galacto-oligosaccharides (GOS) on behavior, the endocrine and immune systems, and gut microbiota [36]. Adult mice were assigned to a control group or one of three treatment groups: FOS, GOS, or combination FOS and GOS. After 3 weeks of treatment, the mice underwent behavioral and cognitive testing, followed by assessments of endocrine, immune, and neurobiological function. A separate cohort of mice also underwent a chronic unpredictable social stress protocol to assess behavioral and physiological changes under chronic stress. Additionally, the gut microbiome was analyzed for changes in composition and SCFA concentration.
Regarding depression-associated behavioral testing, mice in the FOS/GOS group exhibited a significantly decreased immobility time in the tail suspension and forced swim tests when compared to mice in the control group (p < 0.01). Mice in the FOS and GOS groups also exhibited a decreased immobility time in the forced swim test, though this difference was not as drastic as observed in the FOS/GOS group (p < 0.05). With respect to anxiety-associated behavioral testing, FOS/GOS administration significantly increased time in the center of the open field test (p < 0.05). In social behavioral testing, mice in the GOS and FOS/GOS groups also exhibited increased bouts of prosocial behavior in the resident-intruder test (p < 0.05). No significant differences were observed in cognitive or nociceptive assessments between the control and the probiotic groups.
Regarding endocrine testing, mice treated with GOS or FOS/GOS displayed decreased stress-induced corticosterone levels and defecation (p < 0.05). Mice in the FOS/GOS group also exhibited a significant reduction in stress-induced hyperthermia (p < 0.01). With respect to hippocampal and hypothalamic gene expression, expression of BDNF (brain-derived neurotrophic factor), gamma-aminobutyric acid (GABAB1) receptor gene, and GABAB2 receptor gene in the FOS/GOS group were found to be increased (p < 0.05), while expression of Crhr1 (corticotropin-releasing hormone receptor 1) were decreased in the GOS and FOS/GOS groups (p < 0.05). Additionally, FOS administration appeared to increase expression of the N-methyl-d-aspartate receptor 2A subunit, while FOS/GOS administration decreased its expression (p < 0.05). In the hypothalamus, FOS/GOS administration appeared to reduce mRNA levels of glucocorticoid receptor (p < 0.01).
Treatment with prebiotics revealed a number of alterations in relative abundances in gut microbiome composition. All three prebiotic groups displayed significantly higher proportions of Bacteroides and Parabacteroides (p < 0.05). In the FOS/GOS group, increases in Verrucomicrobiaceae and Akkermansia were noted when compared with the control group (p < 0.01) and the other two prebiotic groups (p < 0.05). The FOS group also displayed significant increases in Oscillibacter (p < 0.05). Decreases in Desulfovibrio, Ruminococcus, Allobaculum, Turicibacter, Lactobacillus, and Bifidobacterium were also detected in the prebiotic groups (p < 0.05). Additionally, SCFA concentrations were significantly impacted by prebiotic treatment, with increases in acetate observed with FOS and FOS/GOS treatment (p < 0.05), increases in propionate observed with GOS and FOS/GOS treatment (p < 0.01), and decreases in i-butyrate observed in all three treatment groups (p < 0.05).
Chronic social stress appeared to have significant negative effects on social interactions, long-term memory, anhedonia-like behaviors, and depression-like behaviors. These effects were largely attenuated by administration of FOS/GOS. Additionally, administration of FOS/GOS to mice under chronic social stress resulted in significant decreases in stress-induced hyperthermia and corticosterone levels when compared to stressed mice that were untreated (p < 0.05). Analysis of immunological activity revealed higher concentrations of IL-6 and TNFα in untreated stressed mice, while administration of prebiotics revealed similar levels to those of control mice (p < 0.05). Lastly, stress-induced changes in the cecal microbiome, including a decline in the relative abundance of Bifidobacterium (p < 0.001) and the ratio of Actinobacteria to Proteobacteria, were also abolished by treatment of antibiotics.
From these findings, the authors concluded that administration of the prebiotics FOS, GOS, or a combination of FOS and GOS results in a marked change in behavior and brain chemistry related to anxiety and depression in mice, as well as alterations to the gut microbial community. Mice treated with both FOS and GOS displayed the greatest reductions in anxiety levels and depression-like behavior, which suggests additive effects of combined prebiotic administration. The changes in behavior following prebiotic treatment also correlated with changes in gene expression and monoamine levels. The authors propose that these effects may partially be mediated by changes in SCFAs, which can modulate microglial functions in the CNS and contribute to the development of stress-related depression and anxiety [37, 38]. Furthermore, in the setting of chronic psychosocial stress, administration of the combination of FOS and GOS exerted protective effects on behavior, endocrine, and immunological responses, as well as the gut microbiome. While the potential of prebiotics as nutritional therapeutic agents for anxiety and depression appears promising, the mechanisms by which FOS and GOS modulate behavior and physiological processes are not yet fully known, and further studies will be required to elucidate the process by which prebiotics alter the gut–brain–axis in neuropsychiatric disorders.
Human Studies
Altered Fecal Microbiota in Major Depressive Disorders
Jiang et al. analyzed fecal microbiota compositions in active MDD (A-MDD), responding MDD (R-MDD), and healthy controls (HC) to determine alterations in active episodes of MDD and possible dysbiosis in response to antidepressant treatment [29]. Forty-six patients were recruited and screened by one psychiatrist with the Mini-International Neuropsychiatric Interview for pre-existing psychiatric conditions, and the presence of MDD was verified using the Structured Clinical Interview for the Diagnosis and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV). Severity of disease was determined with Hamilton Depression Rating Scale (HAM-D) and Montgomery-Asberg Depression Rating Scale (MADRS). Severity scores were used to separate A-MDD (HAM-D score ≥ 20) and R-MDD (baseline HAM-D score ≥ 20). On the basis of the results of examination, subjects were divided into an A-MDD group (n = 29) and R-MDD group (n = 17). HC (n = 30) subjects were also selected from the same cohort.
Fecal and serum samples were collected when HAM-D scores were reduced by 50% post-treatment. Surprisingly, analysis of bacterial diversity and richness showed significant increases in bacterial diversity in A-MDD relative to HC as evaluated by the Shannon Index. While it is conventionally considered beneficial to have greater gut microbiome diversity, this diversity is untested in regards to its effects on CNS functions, and may not be universally beneficial. The authors cite studies by Fan et al. and Roger et al. which show increases in microbiome diversity in CNS altered populations such as autism, alluding to a potentially negative impact of increased microbiome diversity [39, 40].
Serum samples were evaluated for inflammatory biomarkers TNFα, IL-1β, IL-6, and BDNF, as inflammation has been associated with dysbiosis and MDD. Notably, serum analysis showed no significant differences between A-MDD, H-MDD, and HC subjects with regards to IL-6, TNFα, and IL-1β. BDNF levels were lower in A-MDD and R-MDD compared to HC. Further studies are needed to determine causation, to better elucidate the role of the gut microbiome in CNS disorders such as MDD.
Short Chain Fatty Acid Profile Alterations in Depressed Polish Women
SCFAs are produced by gut bacteria from dietary fiber. In a study by Skonieczna-Żydeka et al. SCFAs in the stool of depressed and non-depressed women were used as an indicator of microbiome dysbiosis, potentially affecting gut–brain axis signaling as a possible pathogenic cause of depression [41]. A total of 116 Polish women were recruited for this study. Sociodemographic and health-related data were collected by survey. The Beck Depression Inventory (BDI) was used to determine presence and severity of depressive symptoms. BDI scores of up to 11 indicated no depressive symptoms, 12–19 indicated mild depression, 20–25 indicated moderate depression, and 26–63 indicated heavy depression. After evaluation, 35, five, and seven patients were identified to have mild, moderate, and severe depression, respectively. Depressive severity groups were pooled into one depressive symptom group of 47 patients as a result of small sample sizes. Survey results indicated no significant differences in socioeconomic or health statuses. Stool samples were collected during overnight fasting.
Analysis of the stool samples revealed that non-depressive women had higher concentrations of all SCFAs except C6:0. The SCFA isocaproic acid was increased in the depressive group. Fiber intake was measured as a potential factor affecting SCFAs levels. Food frequency questionnaires were used instead of food diaries, and the fiber consumption differed from worldwide recommended values. Women with depression ingested less fiber, though this difference was not significant. Accordingly, fiber intake did not correlate with SCFAs concentration and BDI score.
Breakdown by SCFA type revealed predominantly acetate and propionate populations in both groups. Acetate and propionate showed a negative correlation with severity of depression symptoms. Acetic acid, propionic acid, and caproic acids have been shown to partly contribute to the origin of depressive symptoms through the gut–brain axis. Acetate is described as preventative for enteropathogenic infections and maintains gut barrier integrity, thereby maintaining gut–brain axis signaling. The lower levels of acetate observed in depressive patients cause a decrease in butyric acid. Butyric acid typically inhibits histone deacetylation and prevents hippocampal microglia activation. Decreased butyric acid may cause depressive-like behaviors secondary to neuroinflammation due to increased microglial activation. Of note, many SCFA act as histone deacetylation inhibitors and may contribute to this pathway though to a lesser extent than acetate. Propionate has been demonstrated to dampen innate immune cell response to bacteria, and may also have roles in maintaining proper intestinal permeability [39, 42]. Lower levels may contribute to dysbiosis and neuroinflammation in the CNS leading to depressive symptoms. Through these proposed mechanisms, the authors concluded that SCFAs may partly contribute to women’s emotional health.
Prebiotics, Probiotics, Cytokines, and Cortisol
Dysbiosis and resulting inflammation via cytokine release is one proposed mechanism of MDD, and therefore a potential site for intervention. Kazemi et al. conducted a double-blind, placebo-controlled study to evaluate the effects of probiotics and prebiotics on inflammatory markers and urinary cortisol levels in patients with MDD [43]. A total of 110 patients were recruited and randomly assigned to the prebiotic group (n = 36), the probiotic group (n = 38), and the placebo group (n = 36). Prebiotics were defined as dietary, non-viable food components and probiotics were defined as live microorganisms that, when administered in adequate amounts, confer a health benefit to the host. Baseline testing showed no significant differences between the three groups. Serum cytokine levels for TNFα, IL-1, IL-6, and IL-10 were measured, alongside urine cortisol levels. BDI was used to evaluated depressive symptoms.
There were no statistically significant differences between any groups in cytokine or urine cortisol levels. The authors acknowledge similarly conflicting results in the literature. There is previous evidence that antidepressants may affect gut microbiota by potentially masking possible effects of probiotics and prebiotics on cytokine levels. Even after adjustment for confounding factors, no statistical differences were observed for inflammatory markers. Another reason for the lack of change in cytokine levels may be due to the method of collection. Serum cytokine levels were measured in this study, though serum levels may not account for total cytokine levels in the body.
Urinary cortisol levels did decrease in the probiotic group, though the effect was not statistically significant. BDI scores were improved compared to the placebo group. Scores for the prebiotic group demonstrated no statistical difference. Improvement in BDI scores suggest that depressive symptoms may be improved by probiotic use through mechanisms other than reducing cytokine release. The limitations in this study include a small sample size, a lack of fecal microbiome analyses to account for baseline differences in patients, and a focus on serum cytokine levels. Such limitations can be overcome in larger-scale studies.
Probiotics and Sad Mood Reactivity
Steenbergen et al. evaluated the use of multispecies probiotics in cognitive reactivity scores of healthy patients in a blinded study [44]. Cognitive reactivity has been indicated in the development of depression and has been a target for prevention of depression. In adherence to the conventional theory that dysbiosis leads to increased inflammation from a leaky gut, bacterial strains that improve epithelial barrier function were chosen. Forty healthy patients were recruited and screened with the Mini International Neuropsychiatric Interview (MINI) for pre-existing psychiatric disorders. Then the subjects were randomly assigned to the placebo group (n = 20) or the probiotic group (n = 20). Patients were evaluated by questionnaire for cognitive reactivity, sad mood, and symptoms of depression and anxiety.
The probiotic group showed significantly decreased scores for overall cognitive reactivity, and dramatically reduced scores for the subtypes rumination and aggression. Rumination, or recurrent thoughts about consequences and causes of distress, has been indicated in perpetuating sad moods into depressive episodes. Reduction in rumination may reduce the development of depression. Aggressive thoughts have been associated with suicidal ideation. Reducing suicidal ideation or action is also a positive intervention in depression and may be another benefit for probiotic use.
Biological mechanisms of action for probiotics were not tested in this study, though the authors hypothesized three potential mechanisms. The first hypothesis includes increased serotonin levels. Increasing gut microbiota, especially certain species, has been shown to increase plasma tryptophan levels. Higher tryptophan levels allow for greater synthesis of serotonin. The second hypothesis involves the release of inflammatory cytokines as a major contributor to depression. Probiotics are thought to decrease intestinal epithelial permeability thereby decreasing immune stimulation and release of inflammatory cytokines. The third hypothesis proposed by the authors relates to increased stimulation of the vagus nerve. There is no proposed mechanism of action for this in human studies. A number of animal models have shown vagal stimulation playing a role in depressive and anxiety behaviors. In humans, vagus nerve stimulation has been used successfully as treatment for depression.
Marital Distress, Depression, and Leaky Gut
Chronic, elevated inflammation can predispose individuals to developing an inflammation-related disorder like depression. In a study by Kiecolt-Glaser et al. married couples were evaluated for increases in inflammation markers such as LPS-binding protein (LBP), soluble CD14 (sCD14), and CRP to determine if increased gut permeability is a potential mechanism for marital distress and depression [45]. LBP and CD14 are typically released in response to bacterial translocation of endotoxins and are markers for leaky gut. Forty-three couples (n = 86) were recruited for a double-blind, randomized crossover study, during which the couples received either a high saturated fat or oleic sunflower meal after fasting for 12 h and eating three standardized meals the day prior. Baseline measurements were taken 25 min after catheter placement. Afterwards, the meal was provided to the couple. Two hours later, the couple discussed a marital problem and blood samples were taken every 2 h for 7 h.
A strong, significant correlation was seen between hostile behavior and LBP. A trend of lower sCD14 with more hostile behavior was observed; however, there was no association of sCD14 with mood disorder history. The ratio of LBP/sCD14 was statistically significantly associated with marital satisfaction in patients with a history of mood disorders. Lower marital satisfaction correlated with LBP as well. LBP is a surrogate marker for microbial translocation and typically reflects higher endotoxin levels of gram-negative bacteria since they predominate in the gut. Episodes of dysbiosis are usually transient, and normal gut flora is returned, though prolonged dysbiosis can permanently alter the gut microbiota and can cause changes in the regulation of inflammation, immunity, and gut barrier function. Patients with a history of mood disorder are more susceptible to episodes of dysbiosis due to their chronic inflammatory state, which is reflected in the higher ratio of LBP/sCD14 ratio observed in this study.
CRP was associated with a nonsignificant increased LBP/sCD14 ratio. There was also a nonsignificant trend of higher LBP and sCD14 with IL-6 levels. CRP levels are clinically prognostic, especially when considering risk of cardiovascular disease and events. The elevated CRP seen in this study is likely due to the preference of sedentary, obese couples. CRP may not be directly associated with gut dysbiosis.
Lactobacillus Double-Blind Study
Rudzki et al. sought to assess the psychobiotic and immunomodulatory effects of the probiotic bacteria Lactobacillus plantarum 299v (LP299v) in patients with MDD also being treated with selective serotonin reuptake inhibitors (SSRIs) [46]. They completed a double-blind, placebo controlled study with 79 patients with MDD. Patients were randomized into a placebo group, which received SSRI treatment with placebo probiotic, and a probiotic group, which received SSRI treatment with LP299v probiotic. Sixty patients completed the trial with 30 patients in each group. Severity of psychiatric symptoms, cognitive function, and biochemical parameters were measured.
Results of the study showed decreased kynurenine concentrations (p = 0.005) alongside improved cognitive functions in the probiotic group. Baseline cognitive measurements were taken initially and then repeated at 8 weeks post-intervention. The probiotic group demonstrated significantly improved scores in attention and perceptivity as well as verbal learning tasks as compared to control groups (p = 0.006 and p = 0.023, respectively). Kynurenines have neurotoxic and neurodegenerative effects on CNS. At physiological levels, however, they function to regulate immunomodulation and neuroprotection in the CNS. Proinflammatory cytokines are initiators of the kynurenine synthesis pathway. Several mechanisms for improved cognition and reduced kynurenine were proposed by the authors. One relates to increased intestinal permeability due to physiological stress, leading to low grade inflammation and the production of proinflammatory cytokines. These cytokines initiate the kynurenine pathway, thereby affecting mood and cognition by neurotoxic effects. LP299v is known to reduce gut epithelial permeability, and this function may have reduced levels of kynurenine produced, thereby leading to improved cognition. LP299v adheres to the gut wall and may inhibit growth of other potentially pathogenetic bacteria while also increasing the number of potentially beneficial bacteria. This alteration could enhance SCFA synthesis to also modulate cytokine production.
Another mechanism involves modulation of indoleamine 2,3-dioxygenase (IDO) activity, an immune modulatory enzyme, by hydrogen peroxide activity. LP299v is able to accumulate hydrogen peroxide, and the accumulated hydrogen peroxide inhibits IDO activity, causing downstream inhibition of kynurenine production. The ratio of kynurenine/tryptophan is thought to reflect IDO activity. This study did not show a significant difference in kynurenine/tryptophan ratio between placebo and probiotic groups. This ratio is dependent on available tryptophan and does not measure IDO activity directly. Therefore, this mechanism cannot be ruled out as a possible mechanism for decreased kynurenine and improved cognition.
Synthesis of 5-HT by LP299v and other beneficial gut bacteria can also modulate tryptophan and kynurenine levels. Increased synthesis of 5-HT leads to a decreased level of available tryptophan for kynurenine synthesis. Beneficial gut bacteria also play a major role in producing cofactors necessary for a large variety of biochemical reactions in the body, including kynurenine synthesis. This study observed an increase in vitamin B cofactors associated with kynurenine synthesis and metabolism. Increasing both synthesis and metabolism prevents a buildup of toxic kynurenine and potentially yields improved cognition.
This study was notable for being the first of its kind to demonstrate a link between increased cognitive function and decreased kynurenine concentrations in MDD patients via probiotic supplementation. This provides evidence for a potential role of probiotics in treating some symptoms of MDD and possibly improving cognition in a more general population of patients.
Lactobacillus plantarum and Stress
Lew et al. conducted a 12-week randomized, double-blind, placebo-controlled trial to evaluate the effects of probiotic Lactobacillus plantarum P8 in alleviating stress in adults [47]. Depression Anxiety Stress Scales (DASS-42) and Perceived Stress Scale (PSS-10) surveys were used to determine effects on memory and cognition. Physiological markers were also used to measure glucocorticoid hormone levels in the serum.
Reduced stress scores were observed with DASS-42 survey at week 4, but no difference was seen with the PSS-10 survey. Although both P8 and placebo showed significant reductions in stress and anxiety scores (p = 0.030), the P8 group showed significantly greater improvement in reported stress versus the placebo group (p < 0.05) in DASS-42 surveys given at weeks 4, 8, and 12. The lack of significance seen with the PSS-10 may be due to the different structures of the assessment tools. The PSS-10 tool is a ten-item questionnaire used more frequently in research and focuses on circumstances and situations that may induce anxiety or stress. The DASS-42 in comparison is more robust, being a 42-item survey that is used more frequently in clinical settings and focuses on general feelings of stress and anxiety. Cortisol levels were also found to be marginally different between the groups, though this trend was not statistically significant. This may be due to the diversity of glucocorticoids and their role in many cellular metabolic processes. A narrower target may be needed to establish trends.
Lower plasma pro-inflammatory cytokines interferon gamma (IFNγ) and TNFα were observed alongside improved cognitive and memory potential, as assessed by DASS-42, in the probiotic group. Stress has been shown to alter neuronal morphology and can suppress neuronal proliferation. Synaptic plasticity and firing properties may also be altered. Ultimately, hippocampal volume is reduced, and memory, learning, and cognitive abilities are diminished. Correlational analysis of the proinflammatory cytokines revealed a positive correlation with the psychological traits measured by DASS-42, and psychological traits were correlated with memory and cognition. These correlations indicate that inflammation may help to promote the subjective experience of stress and anxiety, which has been shown to decrease cognitive performance. In the probiotic group, reduced stress and anxiety improved cognition and memory, potentially by targeting these inflammatory pathways. The probiotic Lactobacillus plantarum P8 used in this study has been associated with increased beneficial gut bacteria while inhibiting the growth of potentially harmful gut bacteria, and has increased production of SCFAs in adults, thereby mediating and reducing harmful inflammation associated with stress and dysbiosis.
Probiotics in Postpartum Patients
Postpartum depression and anxiety have few treatments that are safe and effective. To explore the role of probiotics in this population, Slykerman et al. studied the effect of Lactobacillus rhamnosus HN001 (HN001) in pregnancy and postpartum maternal depression and anxiety [48]. This randomized, double blind, placebo-controlled trial was a secondary measure in a study on eczema. Of 423 women in the trial, 380 completed the psychological measure. The experimental group (n = 193) was treated with HN001 daily for 6 months postpartum, while the placebo group (n = 187) underwent daily placebo treatments for 6 months postpartum. Modified versions of the Edinburgh Postnatal Depression Scale and State Trait Anxiety Inventory were used to assess symptoms of depression and anxiety.
The prevalence of scores for depression and anxiety above the cutoff values at 1–2 months postpartum were higher in this study than the 10–15% typically reported. Patients with a history of allergies are known to be at a higher risk for mental problems. As this study was a secondary outcome for families seeking treatment for eczema, the prevalence of depression and anxiety may be higher in this population than the general population. Another possible cause for the increased prevalence is that patients completed the questionnaires regarding depression and anxiety retrospectively. Despite the increased prevalence, the number of women taking psychiatric medications during pregnancy was low, thereby reducing any cofounding factors for study results.
Significantly lower levels of postpartum depression (p = 0.037) and anxiety symptoms (p = 0.014) were reported in the probiotic group. While no mechanism of action was investigated in this study, the authors described two mechanisms of action shown in animal models that may explain their results. For example, in mice treated with L. rhamnosus, changes in the GABA receptors in the brain have been demonstrated alongside anxiety-related behavior. These changes were absent in mice with regions of the vagus nerve removed, indicating a link between the gut and the brain. Another model demonstrated resolution of anxiety behaviors induced by maternal separation by treatment with Bifidobacterium infantis [48].
Infant colic has also been associated with higher depression and anxiety scores, suggesting that probiotic use in infants may benefit maternal mood by reducing infant colic. However, in this study infants were likely only indirectly exposed to small amounts of probiotic. Furthermore the prevalence of infant colic did not differ between probiotic and placebo groups. Multivariate analysis showed that probiotic supplementation and absence of infant colic were independently associated with lower postnatal depression and anxiety scores.
Probiotic vs Prebiotic vs Placebo in Major Depressive Disorders
Akkasheh et al. analyzed the effects of probiotic intake on symptoms of depression and metabolic status in patients with MDD [49]. They conducted a randomized, double blind, placebo-controlled trial of 40 patients with MDD (DSM-IV criteria). Patients were randomly assigned to either a probiotic (n = 20) or placebo group (n = 20). Probiotic supplementation consisted of combination of Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium bifidum.
In the probiotic group, significantly reduced BDI scores were observed compared to placebo, along with a significant decrease in anxiety symptoms. The authors hypothesized that increased levels of tryptophan lead to decreased serotonin metabolite concentrations in the frontal cortex and decreased dopamine levels in the amygdaloid cortex. Probiotics, through fermentation of dietary components, may be able to change the composition or activity of the normal gut flora. This may result in improved peripheral and central nervous system symptoms. Probiotics may also directly influence the enteric and central nervous systems in addition to their mucosal immune system effects.
Decreased serum insulin concentrations and HOMA-IR (homeostatic model assessment of insulin resistance) were also observed in the probiotic group. No significant changes were noted for FPG (fasting plasma glucose), HOMA-B (homeostatic model assessment for beta cells), QUICKI (quantitative insulin-sensitivity check index), or lipid profiles. The literature supports no changes in lipid profile, though the decrease in insulin levels observed in the probiotic group is a unique finding. Insulin reduction may be due to increased hepatic natural killer T cell numbers and a reduction in inflammatory signaling. Linoleic acid is also produced by some species of Lactobacillus, which may upregulate adiponectin and downregulate inflammation to block suppression of GLUT4 transporters.
High sensitivity C-reactive protein (hs-CRP) was also decreased in the probiotic group. Hs-CRP is a marker of systemic inflammation and a predictor of adverse cardiovascular events. The anti-inflammatory effects of probiotics may be due to production of SCFAs in the colon and decreased expression of IL-6. An increase in plasma reduced glutathione (GSH) was also observed in the probiotic group. However, no changes on total antioxidant capacity levels were seen. Although the mechanism of oxidative stress is unknown, the beneficial effects of probiotics on GSH levels might be due to enhanced glutamate–cysteine ligase activity, thereby increasing synthesis of GSH.
Clinical and Metabolic Responses to Probiotics in Major Depressive Disorders
Kazemi et al. conducted a randomized, double blind, placebo-controlled study to compare the effects of probiotic and prebiotic supplementation on the BDI as a primary outcome, and the kynurenine/tryptophan ratio and tryptophan/branched chain amino acids (BCAAs) ratio as secondary outcomes in patients with MDD [43]. A total of 81 patients were enrolled in this study and randomly assigned to probiotic group (n = 28), prebiotic group (n = 27), and placebo group (n = 26). The bacteria used in the probiotic group consisted of Lactobacillus helveticus and Bifidobacterium longum; the prebiotic was galacto-oligosaccharide.
After 8 weeks of treatment, the probiotic group demonstrated a significant decrease in BDI score compared to both prebiotic and placebo groups (p = 0.042). These results were consistent with the literature, though this study is unique in that the use of probiotics was a primary method of treatment. The main mechanisms postulated for the observed probiotic BDI score reduction include modulation of neurotransmitters and inflammation.
Additionally, serum kynurenine/tryptophan ratio was significantly reduced in the probiotic group compared to the placebo group (p = 0.048). The prebiotic group did not show any significant changes. However, this result was only seen when adjusted for serum isoleucine. Tryptophan is metabolized by two main pathways, the serotonin and kynurenine pathways. Shunting of tryptophan towards the production of kynurenine leads to a serotonin deficiency. Probiotics, however, drive tryptophan metabolism down the serotonin pathway. This increase in serotonin may therefore reduce depression and anxiety by increasing the availability of serotonin, much like the mechanism behind SSRIs.
No significant increase in tryptophan/BCAAs ratio was observed in the probiotic group. However, the prebiotic group did show a significant increase in the ratio when compared to the placebo group (p = 0.031). The authors theorize that the significance of this ratio is that BCAAs compete with tryptophan for passage through the blood–brain barrier. BCAAs are produced by some strains of gut bacteria. Notably, probiotics or prebiotics may reduce relative proportions of BCAA, thereby increasing tryptophan entry to the brain and subsequent serotonin production. This could then theoretically decrease symptoms of depression and anxiety. Despite the fact that probiotics were shown to reduce depression in this study, they did not significantly alter the tryptophan/BCAA ratio. Conversely, while prebiotics were able to increase the ratio of these components, prebiotics were not associated with a significant change in depressive symptoms. Though limited in size and scope, the study offers promise in the study of microbiome alterations in treating depression moving forward.
Conclusion
The utilization of microbiome alterations to treat disease remains in its infancy. Though studies exploring its role in various disease processes generally show promise, mechanisms remain unclear and evidence-based treatments for most illnesses have not yet been developed. The animal studies reviewed here offer an excellent array of basic science research that continues to clarify mechanisms by which the microbiome may affect mental health. Moreover, treatment with probiotics or other tools increasingly demonstrate efficacy. The microbiota–gut–brain axis is postulated to modulate various central brain processes as well as production of microbial metabolites and immune mediators that may involve effective changes in cognitive function and behavior. Further evidence is needed, particularly as it relates to translating this work to human subjects and identifying key areas for possible medicinal targets in the treatment of neuropsychiatric disorders. The studies presented in this paper largely demonstrate encouraging results in the treatment of depression, but they are limited by small sample sizes and disparate methodologies, among other factors. Ultimately, there is reason to remain hopeful about what may come to light in the years ahead in microbiome research. There are many potential applications for this area of study and depression is an especially promising area for the role of the microbiome.
References
Frank DN, Pace NR. Gastrointestinal microbiology enters the metagenomics era. Curr Opin Gastroenterol. 2008;24(1):4–10.
Sherwin E, Sandhu KV, Dinan TG, Cryan JF. May the force be with you: the light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs. 2016;30(11):1019–41.
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392.
Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17(5):565–76.
Forsythe P, Kunze WA. Voices from within: gut microbes and the CNS. Cell Mol Life Sci. 2013;70(1):55–69.
Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism—comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11(1):22.
Li C, Cui L, Yang Y, et al. Gut microbiota differs between Parkinson’s disease patients and healthy controls in northeast China. Front Mol Neurosci. 2019;12:171.
Aizawa E, Tsuji H, Asahara T, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254–7.
Xu R, Wu B, Liang J, et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav Immun. 2019. https://doi.org/10.1016/j.bbi.2019.06.039.
Ferrari AJ, Charlson FJ, Norman RE, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders [internet]. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
Bose J, Hedden SL, Lipari RN, Park-Lee E. Key substance use and mental health indicators in the United States: results from the 2017 national survey on drug use and health. 2017. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.
Greenberg PE, Fournier A-A, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(02):155–62.
Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836.
Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1(1):56–66.
Adamsson I, Nord CE, Lundquist P, Sjöstedt S, Edlund C. Comparative effects of omeprazole, amoxycillin plus metronidazole versus omeprazole, clarithromycin plus metronidazole on the oral, gastric and intestinal microflora in Helicobacter pylori-infected patients. J Antimicrob Chemother. 1999;44(5):629–40.
Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280.
Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14.
Bisanz JE, Upadhyay V, Turnbaugh JA, Ly K, Turnbaugh PJ. Meta-analysis reveals reproducible gut microbiome alterations in response to a high-fat diet. Cell Host Microbe. 2019;26(2):265–72.
Ju M, Liu Y, Li M, et al. Baicalin improves intestinal microecology and abnormal metabolism induced by high-fat diet. Eur J Pharmacol. 2019;857:172457.
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–5.
Rodríguez JM, Murphy K, Stanton C, et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Heal Dis. 2015;26:26050.
Cong X, Xu W, Romisher R, et al. Gut microbiome and infant health: brain-gut-microbiota axis and host genetic factors. Yale J Biol Med. 2016;89(3):299–308.
Werbner M, Barsheshet Y, Werbner N, et al. Social-stress-responsive microbiota induces stimulation of self-reactive effector T helper cells. mSystems 2019;4(4):e00292-18.
Tsiaoussis J, Antoniou MN, Koliarakis I, et al. Effects of single and combined toxic exposures on the gut microbiome: current knowledge and future directions. Toxicol Lett. 2019;312:72–97.
Kelly JR, Borre Y, O’Brien C, et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res. 2016;82:109–18.
Hoban AE, Moloney RD, Golubeva AV, et al. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience. 2016;339:463–77.
Provensi G, Schmidt SD, Boehme M, et al. Preventing adolescent stress-induced cognitive and microbiome changes by diet. Proc Natl Acad Sci USA. 2019;116(19):9644–51.
Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94.
Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79.
Desbonnet L, Clarke G, Traplin A, et al. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav Immun. 2015;48:165–73.
Tian P, Wang G, Zhao J, Zhang H, Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J Nutr Biochem. 2019;66:43–51. https://doi.org/10.1016/j.jnutbio.2019.01.007.
Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–56.
Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13(4):240–50.
Yang Y, Cui Y, Sang K, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554(7692):317–22.
Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol Psychiatr. 2017;82(7):472–87. https://doi.org/10.1016/j.biopsych.2016.12.031.
Erny D, Hrabě de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–77.
Réus GZ, Fries GR, Stertz L, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–54.
Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatr. 2016;21(6):738–48. https://doi.org/10.1038/mp.2016.50.
Fan W, Huo G, Li X, Yang L, Duan C. Impact of diet in shaping gut microbiota revealed by a comparative study in infants during the first six months of life. J Microbiol Biotechnol. 2014;24(2):133–43.
Skonieczna-żydecka K, Grochans E, Maciejewska D, et al. Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients. 2018;10(12);1939.
Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: from monoamines to the neurogenesis hypothesis model—are we there yet? Behav Brain Res. 2018;341:79–90.
Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2019;38(2):522–8.
Steenbergen L, Sellaro R, van Hemert S, Bosch JA, Colzato LS. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–64.
Kiecolt-Glaser JK, Wilson SJ, Bailey ML, et al. Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology. 2018;98:52–60.
Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–22.
Lew LC, Hor YY, Yusoff NAA, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr. 2019;38(5):2053–64.
Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017;24:159–65.
Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32(3):315–20.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Alexander Capuco, Ivan Urits, Jamal Hasoon, Rebecca Chun, Brittany Gerald, Jason K. Wang, Hisham Kassem, Anh L. Ngo, Alaa Abd-Elsayed, Thomas Simopoulos, Alan D. Kaye, and Omar Viswanath have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11865615.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Capuco, A., Urits, I., Hasoon, J. et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv Ther 37, 1328–1346 (2020). https://doi.org/10.1007/s12325-020-01272-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01272-7