Abstract
Introduction
Dry eye disease (DED) is commonly encountered in eye clinics and hospitals, and it is therefore very important to understand DED prevalence in outpatients.
Methods
A multicenter, hospital-based cross-sectional study was conducted among outpatients in Japan to ascertain DED prevalence and relationships between DED and patient profiles, including eye disease, DED diagnosis history, and surgical history. DED was diagnosed according to diagnostic criteria of the Asia Dry Eye Society. Patient self-assessment of DED-related subjective symptoms was conducted using the 5-Item Dry Eye Questionnaire (DEQ-5). Tear break-up time was evaluated in subjective symptom-positive patients.
Results
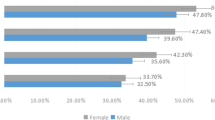
The prevalence of DED was 55.7% in 990 patients (mean age 69.1 ± 13.4 years), DED was commonly experienced in combination with other ocular diseases. In revisiting patients, 15.2% had not previously been diagnosed as DED, and their total DEQ-5 scores were higher than those of patients who had undergone DED treatment.
Conclusion
This study revealed that more than half of the outpatients had DED. Among revisiting patients, there were many “hidden” DED patients who had not been diagnosed with DED in the past. There is a high likelihood of finding DED comorbidity in patients with other eye diseases in eye clinics and hospitals.
Funding
Santen Pharmaceutical Co., Ltd.
Trial Registration
UMIN Clinical Trials Registry Identifier, UMIN000035506.



Similar content being viewed by others
References
Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia dry eye society. Ocul Surf. 2017;15:65–76.
Ridder WH 3rd, Zhang Y, Huang JF. Evaluation of reading speed and contrast sensitivity in dry eye disease. Optom Vis Sci. 2013;90:37–44.
Goto E, Yagi Y, Matsumoto Y, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002;133:181–6.
Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–15.
Uchino M, Uchino Y, Dogru M, et al. Dry eye disease and work productivity loss in visual display users: the Osaka study. Am J Ophthalmol. 2014;157:294–300.
Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90–8.
Castro JS, Selegatto IB, Castro RS, et al. Prevalence and risk factors of self-reported dry eye in Brazil using a short symptom questionnaire. Sci Rep. 2018;8:2076.
Titiyal JS, Falera RC, Kaur M, Sharma V, Sharma N. Prevalence and risk factors of dry eye disease in North India: ocular surface disease index-based cross-sectional hospital study. Indian J Ophthalmol. 2018;66:207–11.
Ferrero A, Alassane S, Binquet C, et al. Dry eye disease in the elderly in a French population-based study (the Montrachet study: maculopathy, optic nerve, nuTRition, neurovAsCular and HEarT diseases): prevalence and associated factors. Ocul Surf. 2018;16:112–9.
Arita R, Mizoguchi T, Kawashima M et al. Meibomian gland dysfunction and dry eye are similar, but different based on a population-based study (Hirado-Takushima study) in Japan. Am J Ophthalmol. 2019 (accepted; in press).
Caffery B, Srinivasan S, Reaume CJ, et al. Prevalence of dry eye disease in Ontario, Canada: a population-based survey. Ocul Surf. 2019;17:526–31.
Yasir ZH, Chauhan D, Khandekar R, Souru C, Varghese S. Prevalence and determinants of dry eye disease among 40 years and older population of Riyadh (except capital), Saudi Arabia. Middle East Afr J Ophthalmol. 2019;26:27–32.
Yu D, Deng Q, Wang J, et al. Air pollutants are associated with dry eye disease in urban ophthalmic outpatients: a prevalence study in China. J Transl Med. 2019;17:46.
Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136:318–26.
Lee TH, Sung MS, Heo H, Park SW. Association between meibomian gland dysfunction and compliance of topical prostaglandin analogs in patients with normal tension glaucoma. PLoS ONE. 2018;13:e0191398.
Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S. Incidence and pattern of dry eye after cataract surgery. PLoS ONE. 2013;8:e78657.
Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156:759–66.
Yokoi N, Uchino M, Uchino Y, et al. Importance of tear film instability in dry eye disease in office workers using visual display terminals: the Osaka study. Am J Ophthalmol. 2015;159:748–54.
Toda I, Shimazaki J, Tsubota K. Dry eye with only decreased tear break-up time is sometimes associated with allergic conjunctivitis. Ophthalmology. 1995;102:302–9.
Onwubiko SN, Eze BI, Udeh NN, Arinze OC, Onwasigwe EN, Umeh RE. Dry eye disease: prevalence, distribution and determinants in a hospital-based population. Cont Lens Anterior Eye. 2014;37:157–61.
Li J, Zheng K, Deng Z, et al. Prevalence and risk factors of dry eye disease among a hospital-based population in southeast China. Eye Contact Lens. 2015;41:44–50.
Ayaki M, Kawashima M, Uchino M, Tsubota K, Negishi K. Possible association between subtypes of dry eye disease and seasonal variation. Clin Ophthalmol. 2017;11:1769–75.
Chalmers RL, Begley CG, Caffery B. Validation of the 5-item dry eye questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye. 2010;33:55–60.
Toda I, Fujishima H, Tsubota K. Ocular fatigue is the major symptom of dry eye. Acta Ophthalmol (Copenh). 1993;71:347–52.
Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–9.
Kawashima M, Uchino M, Yokoi N, et al. The association of sleep quality with dry eye disease: the Osaka study. Clin Ophthalmol. 2016;10:1015–21.
Heimann H, Coupland SE, Gochman R, Hellmich M, Foerster MH. Alterations in expression of mucin, tenascin-c and syndecan-1 in the conjunctiva following retinal surgery and plaque radiotherapy. Graefes Arch Clin Exp Ophthalmol. 2001;239:488–95.
Oh T, Jung Y, Chang D, Kim J, Kim H. Changes in the tear film and ocular surface after cataract surgery. Jpn J Ophthalmol. 2012;56:113–8.
Lee JH, Na KS, Kim TK, Oh HY, Lee MY. Effects on ocular discomfort and tear film dynamics of suturing 23-gauge pars plana vitrectomies. Arq Bras Oftalmol. 2019;82:214–9.
Acknowledgements
We would like to thank all the study participants for their participation and staff of the Fujita Eye Clinic (Tokushima, Japan), Sakka Eye Clinic (Fukuoka, Japan), Kasai Eye Clinic (Tokyo, Japan), Kakinoki Eye Clinic (Tokyo, Japan), Abe Eye Clinic (Oita, Japan), Sapporo Kato Eye Clinic (Hokkaido, Japan), and Heisei Eye hospital (Miyagi, Japan). We also would like to thank RPM Co., Ltd. (Tokyo, Japan) and KONDO Photo Process Co., Ltd. (Osaka, Japan) for their assistance in data management and statistical analysis.
Funding
This study and publication charges were funded by Santen Pharmaceutical Co., Ltd., Osaka, Japan. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Authorship Contribution
Conceptualization, Jun Shimazaki., Hirotsugu Kishimoto and Yoshiaki Yamada; Methodology, Jun Shimazaki., Hirotsugu Kishimoto and Yoshiaki Yamada; Investigation, Yuya Nomura, Shinichiro Numa, Yoko Murase, Kazukuni Kakinoki, Fumihide Abe, Yuji Kato, and Hitoshi Okabe; Project administration, Hirotsuga Kishimoto and Yoshiaki Yamada; Resources, Yuya Nomura, Shinichiro Numa, Yoko Murase, Kazukuni Kakinoki, Fumihide Abe, Yuji Kato, and Hitoshi Okabe; supervision, Jun Shimazaki; Writing—original draft preparation, Yoshiaki Yamada; Writing—review & editing, Jun Shimazaki., Hirotsugu Kishimoto.
Disclosures
Jun Shimazaki has received consulting fees from Santen Pharmaceutical Co., Ltd. about this work. Jun Shimazaki has received consulting fees from QD Laser, Inc., and IDD Inc. outside this work. Jun Shimazaki has received honoraria from Santen Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Senju Pharmaceutical Co., Ltd., and Alcon Pharmaceuticals Ltd. outside this work. Yuya Nomura has received consulting fees from Santen Pharmaceutical Co., Ltd. outside this work. Shinichiro Numa has received consulting fees from Santen Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Kowa Company, Ltd. outside this work. Yuji Kato has received consulting fees, and honoraria from Santen outside this work. Hitoshi Okabe has received consulting fees from Santen Pharmaceutical Co., Ltd., and honoraria from Santen Pharmaceutical Co., Ltd., Sucampo Pharmaceutical Inc., Otsuka Pharmaceutical Co., Ltd., Alcon Japan Ltd., Kowa Company, Ltd., Pfizer Japan Inc., Senju Pharmaceutical Co., Ltd., and Nitten Pharmaceutical Co., Ltd. outside this work. Hitoshi Okabe is an employee at Santen Pharmaceutical Co., Ltd. Yoko Murase, Kazukuni Kakinoki, and Fumihide Abe declare no conflict of interest. Yoshiaki Yamada is an employee at Santen Pharmaceutical Co., Ltd. The funders had no role in the collection, analyses. All facilities have received a grant from Santen towards this work. The following facilities have received grants from Santen Pharmaceutical Co., Ltd. outside this work: Department of Ophthalmology, Tokyo Dental College, Ichikawa General Hospital, wherein Jun Shimazaki is the director; Sakka Eye Clinic, wherein Shinichiro Numa is the director; Kakinoki Eye Clinic, wherein Shinichiro Numa is the director; Abe Eye Clinic, wherein Fumihide Abe is the director; Sapporo Kato Eye Clinic, wherein Yuji Kato is the director, and Heisei Eye Hospital, wherein Hitoshi Okabe is the director. Department of Ophthalmology, Tokyo Dental College, Ichikawa General Hospital has received grants from HOYA corporation outside this work. Abe Eye Clinic has received grants from Wakamoto Pharmaceutical Co., Ltd. outside this work.
Compliance with Ethics Guidelines
This study protocol was approved by The Ethics Committee of Medical Corporation TOUKEIKAI Kitamachi Clinic (Tokyo, Japan). This study was conducted in accordance with the guidelines of the World Medical Association Declaration of Helsinki, and Ethical Guidelines for Medical and Health Research involving Human Subjects in Japan. The subjects received a full explanation of the procedures and provided their written informed consent for participation prior to inclusion in the study. The study was registered in the public registration system (University Hospital Medical Information Network: UMIN (registries no. UMIN000035506)).
Data Availability
The data sets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.10008401.
Rights and permissions
About this article
Cite this article
Shimazaki, J., Nomura, Y., Numa, S. et al. Prospective, Multicenter, Cross-Sectional Survey on Dry Eye Disease in Japan. Adv Ther 37, 316–328 (2020). https://doi.org/10.1007/s12325-019-01143-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01143-w