Abstract
Introduction
Interstitial lung disease (ILD) is a common manifestation of scleroderma/systemic sclerosis (SSc). However, the direct and indirect economic burdens of SSc-ILD remain unclear. This study assessed and compared healthcare resource utilization (HRU), direct healthcare costs, work loss, and indirect costs between patients with SSc-ILD and matched controls with neither SSc nor ILD in the USA.
Methods
Data were obtained from a large US commercial claims database (2005–2015). Patients (at least 18 years old) had at least one SSc diagnosis in the inpatient (IP) or emergency room (ER) setting or at least two SSc diagnoses in another setting, and at least one diagnosis of ILD in the IP or ER setting or at least two diagnoses of ILD in another setting. Controls with neither SSc nor ILD were matched 5:1 to patients with SSc-ILD. Comparisons were conducted using Wilcoxon signed-rank and McNemar’s tests and adjusted odds ratios (ORs) and incidence rate ratios (IRRs).
Results
A total of 479 SSc-ILD patients and 2395 matched controls were included (52 SSc-ILD patients and 260 matched controls for work loss and indirect cost analyses). Patients with SSc-ILD had significantly higher HRU and costs, IP admissions (adjusted IRR = 5.6), IP hospitalization days (adjusted IRR = 12.0), ER visits (adjusted IRR = 2.8), OP visits (adjusted IRR = 3.1), and days of work loss (adjusted IRR = 4.5). The adjusted difference in annual direct healthcare costs was $28,632 (SSc-ILD, $33,195; controls, $4562) and that in indirect costs was $4735 (SSc-ILD, $5640; controls, $906) (all p < 0.0001).
Conclusion
SSc-ILD patients had significantly higher HRU, work loss, and direct and indirect costs compared to matched controls with neither SSc nor ILD.
Funding
Boehringer Ingelheim Pharmaceuticals, Inc.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Scleroderma, also known as systemic sclerosis (SSc), is a multisystem, chronic, autoimmune disease that manifests itself with varying degrees of progressive fibrosis, vasculopathy, and inflammation of the skin and internal organs, including the gastrointestinal tract, heart, kidneys, and lungs [1]. On the basis of the pattern of skin involvement, SSc is typically classified as either limited SSc, when it is confined to the skin distal to the elbows and knees, or diffuse SSc, when it extends to the skin of the proximal extremities and trunk [2, 3]. While organ involvement can be present in both forms of SSc, patients with diffuse SSc are at a higher risk of organ dysfunction [3, 4]. Worldwide, SSc has a prevalence of 50–300 per million [5, 6] and is more often observed in women between the ages of 20 and 50 years [4, 7]. In the USA, SSc is estimated to affect up to 240 people per million [5, 6].
One of the most common manifestations of SSc is interstitial lung disease (ILD), a group of chronic lung conditions affecting the pulmonary interstitium. The presence of ILD in SSc (SSc-ILD) is commonly diagnosed using high-resolution computed tomography (HRCT), chest x-ray, and biopsy [8,9,10]. Although estimates vary significantly on the basis of method of detection, between 30% and 90% of patients with SSc are believed to develop ILD over the course of the disease [11]. Symptoms of ILD typically include dry cough, fatigue, and dyspnea, which may exacerbate pre-existing SSc-related complications, such as lung fibrosis [9]. ILD is a leading cause of morbidity and mortality among patients with SSc [12, 13]. Furthermore, lung involvement has been linked to a substantial increase in healthcare costs among patients with SSc [14, 15]. In one recent US study, over a 5-year period, mean all-cause healthcare costs for patients with SSc-ILD were estimated at $191,107 (2014 USD) [15]. Nevertheless, there is a paucity of studies assessing the direct economic burden of illness of SSc-ILD in the USA. Moreover, no studies evaluating the indirect economic burden of illness of SSc-ILD in the USA have been identified in the literature.
To address this knowledge gap, the present study sought to characterize the burden of illness of SSc-ILD in the USA. To that end, a large nationwide administrative claims database was used to quantify and compare healthcare resource utilization (HRU), direct healthcare costs, work loss, and indirect costs during 1 year after SSc-ILD diagnosis between patients with SSc-ILD and matched controls in the USA.
Methods
Data Source
This study used data from the OptumHealth Care Solutions, Inc. database covering 84 self-insured Fortune 500 companies. The database includes medical and pharmaceutical claims for over 19 million privately insured individuals and their families, across industries, job classifications, and regions in the USA. For the employees of 44 of the 84 companies in the database, short- and long-term disability claims are also available. The data were de-identified and fully compliant with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act. No institutional review board approval was required. Data from Q3 2004 to Q1 2016 were used for this study.
Sample Selection and Study Design
Patients with SSc-ILD
To be eligible for inclusion, patients with SSc-ILD were required to have (1) at least one medical claim with a diagnosis of SSc [International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 710.1] in the inpatient (IP) or emergency room (ER) setting or at least two medical claims with a diagnosis of SSc in the outpatient (OP) or other settings on two different dates between January 1, 2005 and March 31, 2015; and (2) at least one medical claim with a diagnosis of ILD (ICD-9-CM code 515, 516.0, 516.3, 516.4, 516.5, 516.8, 516.9, 517.2, 517.8, 518.89) in the IP or ER setting or at least two medical claims with a diagnosis of ILD in the OP or other settings on two different dates between January 1, 2005 and March 31, 2015. For patients with SSc-ILD, the index date was defined as the later date of the first SSc diagnosis and the first ILD diagnosis between January 1, 2005 and March 31, 2015. To be included in the study, patients were additionally required to be at least 18 years old at the index date and have continuous healthcare plan enrollment in the 6-month period preceding the index date (baseline period). In addition, for HRU and direct healthcare cost analyses, patients were required to have continuous healthcare plan enrollment for 12 months following the index date (follow-up period for HRU and direct healthcare costs, hereafter referred to as 12-month follow-up period). For work loss and indirect cost analyses, patients were required to be primary policy holders and actively employed (i.e., not retired) on the index date and to have continuous healthcare plan enrollment for 6 months following the index date (follow-up period for work loss and indirect costs, hereafter referred to as 6-month follow-up period). The duration of the follow-up for the indirect cost and work loss analyses was set as the first 6 months after the index date (versus the first 12 months, used for HRU and direct healthcare cost analyses) to ensure a sufficiently large sample size was available.
All patients were required to have neither claims with a diagnosis of SSc nor ILD during the baseline period. Finally, patients were also required to have a chest HRCT (Current Procedural Terminology (CPT) code 71270) or chest computed tomography (CT; CPT code 71250, 71260) within 90 days of the first ILD diagnosis between January 1, 2005 and March 31, 2015.
Matched Controls
Controls were matched with a ratio of 5:1 to patients with SSc-ILD so that for each case of SSc-ILD there were five matched controls. All controls were required to have neither a diagnosis of SSc nor ILD in their entire claims history. This matching ratio was used to increase the accuracy of the analysis given the small expected sample size of the SSc-ILD patient group. The index date for the matched controls was selected as the same calendar date as that of the patients with SSc-ILD to whom they were matched. Controls were matched to patients with SSc-ILD on age at the index date, sex, region of residence (i.e., Northwest, Midwest, South, West, unknown), insurance type (i.e., preferred provider organization (PPO), point of service (POS), indemnity, unknown/other), and length of follow-up (for direct healthcare costs and HRU analyses). The matched controls were additionally required to have continuous healthcare plan enrollment during the 6-month baseline period and the 12-month follow-up period (6 months for indirect burden assessment).
Study Measures
Baseline Characteristics
Patient characteristics measured during the baseline period included demographics (e.g., age, gender) at the index date, the index year (the year of the index date), a modified Charlson comorbidity index (CCI) [16] and selected CCI components, in which diagnoses of SSc were excluded from the rheumatic disease component of the CCI, and SSc and non-SSc-related comorbidities. During the baseline period, all-cause HRU and healthcare costs (i.e., medical and pharmacy) were also reported.
Healthcare Resource Utilization and Clinical Outcomes in the 12-Month Follow-up Period
For both patients with SSc-ILD and matched controls, all-cause HRU measures during the 12-month follow-up period included IP admissions and total IP hospitalization days, ER visits, OP visits, specialist visits, and other visits (including lab tests and medical services at home). In addition, select procedures, medication use, and SSc-related comorbidities, as well as the modified CCI and individual components of the modified CCI were also measured.
Direct Healthcare Costs in the 12-Month Follow-up Period
Direct healthcare costs during the 12-month follow-up period included medical and pharmacy costs. All-cause healthcare costs were estimated for both patients with SSc-ILD and matched controls and included IP, ER, and OP costs as well as costs for other visits (including lab tests and medical services at home).
All direct healthcare costs were inflated to 2018 US dollars (USD).
Work Loss in the 6-Month Follow-up Period
Work loss analyses included a subset of patients with available work loss data. Work loss during the 6-month follow-up period included (1) total days of work loss due to disability; (2) days of medically related absenteeism, defined as the business days (Monday to Friday) in which medical services (e.g., hospital IP visits) were used as well as the days after the injury date (i.e., the date of the injury that results in the disability claim; obtained from medical claims) were used and before the start of disability (based on disability claims). Each hospitalization day and emergency department visit was assumed to account for a full day of work loss; each OP or other visit was assumed to account for half a day of work loss.
Indirect Costs in the 6-Month Follow-up Period
Indirect costs during the 6-month follow-up period were estimated for patients with available work loss data and included medically related absenteeism costs and disability costs. Medically related absenteeism costs were based on the wage information of individual employees reported in eligibility files and on days of medically related absenteeism, as defined above. Disability costs during the 6-month follow-up period were estimated for patients with available work loss data and for those with any disability; they were based on the wage information of individual employees reported in eligibility files and on days of disability, which were identified using disability claims.
All indirect costs were inflated to 2018 USD.
Statistical Analysis
For baseline characteristics and clinical outcomes during the follow-up period, mean and standard deviation (SD) were reported for continuous variables while frequency and percentages were reported for categorical variables. Comparisons were made between patients with SSc-ILD and matched controls using generalized estimating equations to account for correlation within matched controls for continuous and categorical variables.
For HRU and work loss, the proportions of patients with any event and mean event counts were described during the 12- and 6-month follow-up periods, respectively, and compared between patients with SSc-ILD and matched controls using generalized estimating equations to account for correlation within matched controls for continuous and categorical variables. In multivariable analyses, adjusted odds ratios (ORs) were calculated using generalized estimating equations with binomial distributions while adjusted incidence rate ratios (IRRs) were calculated using Poisson distributions accounting for overdispersion, controlling for baseline covariates, including age, sex, index year (i.e., 2005–2010 vs. 2011–2015), region of residence (i.e., South vs. other), insurance type (i.e., PPO vs. other), industry category (i.e., manufacturing, transportation, government vs. other), and selected baseline comorbidities that were not related to SSc and with prevalence greater than 5% in any cohort (i.e., diabetes without chronic complication, any malignancy including leukemia and lymphoma). As a chronic condition, symptoms can be present long before diagnosis is given, so HRU related to SSc-ILD may have occurred during the baseline period. Characteristics associated with SSc or ILD, such as SSc-related comorbidities, baseline HRU, or baseline costs, were not included as control variables; these variables may reflect pre-index burden of SSc-ILD, so adjusting for SSc-related or ILD-related characteristics could introduce bias.
For direct healthcare and indirect costs, unadjusted costs during the 12- and 6-month follow-up periods, respectively, were compared between patients with SSc-ILD and matched controls using generalized estimating equations to account for correlation within matched controls. In multivariable analyses, mean adjusted costs were compared between patients with SSc-ILD and matched controls using generalized estimating equations with Tweedie distributions to account for the matched design controlling for the same covariates as those listed above for HRU and work loss.
Compliance with Ethics Guidelines
The New England IRB (NEIRB) has determined that this study is exempt from ethics approval as only existing data were used and patient data could not be identified, directly or through identifiers linked to the subjects. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was not applicable to this study.
Results
Baseline Period
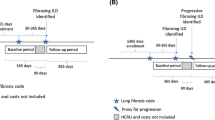
A total of 479 patients with SSc-ILD and 2395 matched controls met the inclusion criteria (Fig. 1); of these, 52 patients with SSc-ILD and 260 matched controls were included in the work loss and indirect cost analyses (Fig. 1).
Sample selection. CT computed tomography, HRCT high-resolution computed tomography, ILD interstitial lung disease, SSc systemic sclerosis. aIndex date was defined as the later date of the first SSc diagnosis and first ILD diagnosis between January 1, 2005 and March 31, 2015. bThe baseline period was defined as the 6-month period before the index date. cThe follow-up period was defined as the 12-month period following the index date. dOne patient was dropped in the last step, because the patient could not be matched to five controls. ePatients were eligible for work loss analyses if they were primary policyholders and were actively employed on the index date with at least 6 months of post-index eligibility in their health plan
Table 1 reports baseline characteristics, both matched (i.e., age, sex, region of residence and insurance plan at the index date, and employment industry) and unmatched. For both patients with SSc-ILD and matched controls, mean age at the index date was 60.2 years and 80.6% were female. Patients with SSc-ILD had a significantly higher modified CCI (excluding SSc) than matched controls (1.5 vs. 0.2, p < 0.0001; Table 1). For patients with SSc-ILD, the most common SSc-related comorbidities were chronic pulmonary disease and rheumatic disease (excluding SSc), while the most common non-SSc-related comorbidity was any malignancy including leukemia and lymphoma. Both SSc and non-SSc-related comorbidities were significantly more frequent among patients with SSc-ILD than matched controls (SSc-related: chronic pulmonary disease 39.0% vs. 4.2%, rheumatic disease excluding SSc 24.6% vs. 1.5%; non-SSc-related: any malignancy including leukemia and lymphoma, 9.6% vs. 3.5%; all p < 0.0001; Table 1).
At baseline, patients with SSc-ILD had significantly higher all-cause HRU measures and all-cause costs than their matched controls (all p < 0.0001, Table 1). Total costs were $16,599 for patients with SSc-ILD and $2172 for matched controls (p < 0.0001); of these, medical costs accounted for $13,159 and $1440 for patients with SSc-ILD and matched controls, respectively; while pharmacy costs accounted for $3440 and $732 for patients with SSc-ILD and matched controls, respectively.
Healthcare Resource Use and Clinical Outcomes During the 12-Month Follow-up Period
During the 12-month follow-up period, unadjusted measures of clinical outcomes and medication use were generally higher among patients with SSc-ILD than matched controls (Table 2). Unadjusted and adjusted HRU measures were all significantly higher among patients with SSc-ILD than among matched controls (Table 2 and Table S1 in the electronic supplementary material). For instance, in the adjusted analysis, patients with SSc-ILD had significantly higher odds of IP admissions (43.4% vs. 8.5% for SSc-ILD vs. matched controls; OR 8.5; 95% CI 6.7–10.9), ER visits (39.5% vs. 17.5%; OR 3.0; 95% CI 2.4–3.7) than their matched controls (all p < 0.0001; Table 2). Also, in the adjusted analysis, patients with SSc-ILD had significantly more IP admissions (1.1 vs. 0.2; IRR 5.6; 95% CI 4.8–6.6), total IP hospitalization days (7.2 vs. 0.5; IRR 12.0; 95% CI 10.2–14.2), ER visits (0.8 vs. 0.3; IRR 2.8; 95% CI 2.4–3.3), OP visits (31.8 vs. 9.8; IRR 3.1; 95% CI 2.9–3.4), and other visits (5.2 vs 0.7; IRR 7.0; 95% CI 6.1–8.1) than their matched controls (all p < 0.0001; Table 2).
Direct Healthcare Costs During the 12-Month Follow-up Period
Unadjusted direct healthcare costs during the 12-month follow-up period and indirect costs during the 6-month follow-up period were all significantly higher among patients with SSc-ILD than their matched controls. Total direct healthcare costs were $37,505 and $4997 for patients with SSc-ILD and their matched controls, respectively; of these, medical costs were $27,094 for patients with SSc-ILD and $3437 for matched controls (all p < 0.0001).
Adjusted direct healthcare costs during the 12-month follow-up period were all significantly higher among patients with SSc-ILD than their matched controls (Table 3). The direct healthcare cost difference between patients with SSc-ILD and their matched controls was $28,632 (SSc-ILD, $33,195; matched controls, $4562) for total costs, $23,307 (SSc-ILD, $25,977; matched controls, $2670) for total medical costs, and $7475 (SSc-ILD, $8929; matched controls, $1455) for pharmacy costs (all p < 0.0001; Table 3).
Work Loss During the 6-Month Follow-up Period
During the 6-month follow-up period, for all outcomes for which the regression models converged, patients with SSc-ILD had significantly higher unadjusted and adjusted work loss measures than their matched controls (Table 4). For instance, in the adjusted analysis, patients with SSc-ILD had significantly more total days of work loss (23.2 vs. 4.8; IRR 4.5; 95% CI 2.9–7.0), including days of disability (14.8 vs. 2.2; IRR 9.0; 95% CI 4.5–18.1) and days of medically related absenteeism (8.4 vs. 2.6; IRR 3.2; 95% CI 2.4–4.2) (all p < 0.0001) (Table 4).
Indirect Costs During the 6-Month Follow-up Period
Total unadjusted indirect costs were $6763 for patients with SSc-ILD and $917 for matched controls (all p < 0.0001). Adjusted indirect cost differences during the 6-month follow-up period were all significantly higher among patients with SSc-ILD than their matched controls (Table 3). The indirect cost differences were $4735 (SSc-ILD, $5640; matched controls, $906) for total costs, $3596 (SSc-ILD, $3886; matched controls, $290) for disability costs, and $1602 (SSc-ILD, $2232; matched controls, $630) for medically related absenteeism costs (all p < 0.0001; Table 3).
Discussion
ILD is one of the most common and disabling comorbidities of SSc as well as a leading cause of death among patients with SSc [1, 12, 17]. Nevertheless, its impact on the burden of illness of SSc has not been comprehensively investigated. Accordingly, using claims data, this real-world study assessed the HRU, work loss, and direct healthcare and indirect costs incurred by patients with SSc-ILD in the USA.
Results showed that patients with SSc-ILD incurred a greater direct and indirect economic burden of illness than their matched controls with neither SSc nor ILD. Specifically, patients with SSc-ILD were significantly more likely to have an IP admission or an ER or OP visit than their matched controls. SSc-ILD was also associated with significantly more days of disability and days of medically related absenteeism. In terms of costs, patients with SSc-ILD incurred significantly higher adjusted total direct healthcare costs ($33,195 vs. $4562 for SSc-ILD vs. matched controls; cost difference, $28,632), driven by higher total medical costs ($25,977 vs. $2670; cost difference, $23,307), and significantly higher total indirect costs ($5640 vs. $906; cost difference, $4735) than their matched controls.
These results contrast with those reported in Zhou et al. [18], a study conducted using the same data source but in the overall SSc patient population rather than the SSc-ILD subpopulation. In that study, patients with SSc incurred adjusted total direct healthcare costs of $18,910 versus those of matched controls of $5494; this resulted in a cost difference of $13,416, which is roughly half that reported in the present study for patients with SSc-ILD (after inflation to 2018 USD). A similar pattern was observed for indirect costs: incremental indirect costs for patients with SSc were $3247 (patients with SSc, $4766; matched controls, $1519; after inflation to 2018 USD), which are roughly two-thirds of those reported in the present study for patients with SSc-ILD. Since, in the Zhou et al. study [18], both patients with SSc with and without ILD were included, the actual cost difference between patients with SSc alone and those with SSc-ILD is expected to be larger than the one reported in Zhou et al. [18]. Additional research is needed to further compare the economic burden of illness in patients with SSc alone vs. patients with SSc-ILD.
The results from the present study are in line with those of Fischer et al. [15], the only other previous study that reported all-cause healthcare (medical and pharmacy) costs associated with SSc-ILD. In their analysis—which, unlike ours, did not use a matched-controlled design—Fischer et al. [15] considered claims data from 2003 to 2014 and found that the unadjusted healthcare costs incurred by patients with SSc-ILD during the first year after an SSc-ILD diagnosis were $39,268 (after inflation to 2018 USD), similar to the total direct healthcare costs estimated in the current study ($37,505). Despite a small difference in the results, both studies point to a substantial direct economic burden of illness of SSc-ILD during the first year after a diagnosis of SSc-ILD. It is worth noting that the direct economic burden of SSc reported by Fischer et al. [15] of $23,281, while high, is only roughly 60% of that of SSc-ILD ($39,268; after inflation to 2018 USD). While comparisons were not conducted across cohorts, the difference in burden between patients with SSc and patients with SSc-ILD may point to a substantial impact of ILD on the direct burden of illness.
Indirect costs and work loss were found to be substantial in the first 6 months following the index date. As a sensitivity analysis, summary statistics were calculated in the second 6 months of the first year after diagnosis for indirect cost and work loss analyses, but no comparisons were made as patients with SSc-ILD were not matched to controls for this period. In the second 6 months of the first year after diagnosis, 39 patients met all the eligibility criteria and among them, each measure decreased compared to the first 6 months. For example, total indirect costs decreased from $25,985 to $11,089 and total days of work loss decreased from 23.2 to 15.9. These reductions were likely due to changes in the sample population (e.g., patients with the most work loss and highest indirect costs may not continue employment after 6 months, may not have survived past 6 months, may have become disabled, or may have lost insurance coverage); another possible reason is the fact that the sample size in the second half of the first year post diagnosis was too small for the results to be comparable to the whole SSc-ILD population. While overall these results suggest that patients with SSc-ILD have substantial indirect costs and work loss, additional studies with longer follow-up periods and larger sample sizes may be warranted to study this relationship further.
When interpreting the results of this study, some limitations should be considered. First, given the lack of availability of clinical information in claims data, such as disease severity, the impact of SSc-ILD severity on the economic burden of illness of SSc-ILD could not be assessed; further real-world studies are warranted to evaluate this point. Second, the burden of illness was assessed during the first year after the later date of the first observed diagnosis of SSc and ILD. As such, this study may not capture the full disease burden of SSc-ILD as the onset of the disease may have occurred before the later date of the two diagnoses or even before either diagnosis; this may have led to underestimation of the overall burden of illness of SSc-ILD. Third, this study assumed that each hospitalization day and ER visit accounted for a full day of work loss, while each OP or other visit accounted for half a day of work loss, which may not reflect the true work loss incurred by patients. Moreover, analysis of work loss did not include retired patients or controls. Fourth, given the retrospective observational nature of this study, associations but not causal relationships should be inferred. Fifth, because the data used in this study are limited to commercially insured patients, the results may not be generalizable to the population beyond the data source, such as patients without insurance coverage or with public insurance plans (i.e., Medicaid or Veteran’s Administration plans). In addition, patients who are over 65 may be partially covered by Medicare and therefore not have full coverage from the commercial plans offered by their employers; as a result, their cost data may have been incomplete in the database, possibly underestimating HRU and costs incurred by elderly patients. Lastly, unadjusted and adjusted indirect cost and work loss analyses focused only on the first 6 months after a diagnosis of SSc-ILD to maintain a sufficiently large sample size, so annual indirect costs could not be discerned. Further studies with larger sample sizes are warranted to generalize the results found in the current study.
Conclusions
The results of this claims data study showed that SSc-ILD is associated with a substantial direct economic burden of illness, as evidenced by higher HRU and all-cause healthcare costs compared to matched controls with neither SSc nor ILD. Patients with SSc-ILD also sustained a considerable indirect economic burden as they experienced significantly higher indirect costs due to disabilities and medically related absenteeism as well as significantly more work loss than their matched controls. These results indicate the existence of high unmet needs for patients with SSc-ILD; more effective disease management is needed to help improve patient outcomes and reduce burden of illness.
References
Denton C, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–99.
UpToDate. Diagnosis and differential diagnosis of systemic sclerosis (scleroderma) in adults. 2017. http://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-systemic-sclerosis-scleroderma-in-adults. Accessed Dec 2017.
Johns Hopkins Medicine. Types of Scleroderma. 2017. https://www.hopkinsscleroderma.org/scleroderma/types-scleroderma/. Accessed Dec 2017.
Cliveland Clinic. Systemic Scleroderma. 2010. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/rheumatology/systemic-sclerosis/. Accessed Dec 2017.
Chifflot H, Fautrel B, Sordet C, Chatelus E, Sibilia J. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37:223–35.
Mayes MD. Scleroderma epidemiology. Rheum Dis Clin North Am. 2003;29:239–54.
Alba MA, Velasco C, Simeón CP, et al. Early- versus late-onset systemic sclerosis: differences in clinical presentation and outcome in 1037 patients. Medicine. 2014;93:73–81.
Meyer KC. Diagnosis and management of interstitial lung disease. Transl Respir Med. 2014;2:4–17.
Schoenfeld SR, Castelino FV. Interstitial lung disease in scleroderma. Rheum Dis Clin N Am. 2015;41:237–48.
Orens JB, Kazerooni EA, Martinez FJ, et al. The sensitivity of high-resolution CT in detecting idiopathic pulmonary fibrosis proved by open lung biopsy: a prospective study. Chest. 1995;108:109–15.
Cottrell TR, Wise RA, Wigley FM, Boin F. The degree of skin involvement identifies distinct lung disease outcomes and survival in systemic sclerosis. Ann Rheum Dis. 2014;73:1060–6.
Rubio-Rivas M, Royo C, Simeon CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44:208–19.
Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003.
Furst DE, Fernandes AW, Iorga SR, Greth W, Bancroft T. Annual medical costs and healthcare resource use in patients with systemic sclerosis in an insured population. J Rheumatol. 2012;39:2303–9.
Fischer A, Kong AM, Swigris JJ, Cole AL, Raimundo K. All-cause healthcare costs and mortality in patients with systemic sclerosis with lung involvement. J Rheumatol. 2018;45:235–41.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9.
Saketkoo LA, Magnus JH, Doyle MK. The primary care physician in the early diagnosis of systemic sclerosis: the cornerstone of recognition and hope. Am J Med Sci. 2014;347:54–63.
Zhou Z, Yu Y, Tang W, et al. Economic burden of illness among commercially insured patients with systemic sclerosis in the United States. 2018. http://web.aimgroupinternational.com/2018/sclerosiscongress/wordpress/wp-content/uploads/2018/02/FinalProgramme0602-2.pdf. Accessed Apr 2018.
Acknowledgements
Funding
Sponsorship for this study including article processing charges and the open access fee were funded by Boehringer Ingelheim Pharmaceuticals, Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All authors contributed to the design of the study, the collection, analysis, and interpretation of the data, and manuscript development. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Medical writing assistance was provided by Cinzia Metallo, Ph.D., an employee of Analysis Group, Inc. Support for this assistance was funded by Boehringer Ingelheim Pharmaceuticals, Inc.
Disclosures
Zhou Zhou is an employee of Analysis Group. Darren Thomason is an employee of Analysis Group. Wenxi Tang is an employee of Analysis Group. Xinyue Liu is an employee of Analysis Group. Zheng-Yi Zhou is an employee of Analysis Group. Dendy Macaulay is an employee of Analysis Group. Analysis Group has received consultancy fees from Boehringer Ingelheim Pharmaceuticals for this study. Yanni Fan is an employee of Boehringer Ingelheim Pharmaceuticals, Inc. Aryeh Fischer received personal and consultancy fees from Boehringer Ingelheim Pharmaceuticals, Inc., and F. Hoffmamn-La Roche & Co.
Compliance with Ethics Guidelines
The New England IRB (NEIRB) has determined that this study is exempt from ethics approval as only existing data were used and patient data could not be identified, directly or through identifiers linked to the subjects. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was not applicable to this study.
Data Availability
The data that support the findings of this study are available from OptumHealth Care Solutions, Inc. but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Boehringer Ingelheim Pharmaceuticals, Inc., and OptumHealth Care Solutions, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7825709.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhou, Z., Fan, Y., Thomason, D. et al. Economic Burden of Illness Among Commercially Insured Patients with Systemic Sclerosis with Interstitial Lung Disease in the USA: A Claims Data Analysis. Adv Ther 36, 1100–1113 (2019). https://doi.org/10.1007/s12325-019-00929-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00929-2