Abstract
Introduction
Diuretic responsiveness in patients with chronic heart failure (CHF) is better assessed by urine production per unit diuretic dose than by the absolute urine output or diuretic dose. Diuretic resistance arises over time when the plateau rate of sodium and water excretion is reached prior to optimal fluid elimination and may be overcome when hypertonic saline solution (HSS) is added to high doses of furosemide.
Methods
Forty-two consecutively hospitalized patients with refractory CHF were randomized in a 1:1:1 ratio to furosemide doses (125 mg, 250 mg, 500 mg) so that all patients received intravenous furosemide diluted in 150 ml of normal saline (0.9%) in the first step (0–24 h) and the same furosemide dose diluted in 150 ml of HSS (1.4%) in the next step (24–48 h) as to obtain 3 groups as follows: Fourteen patients receiving 125 mg (group 1), fourteen patients receiving 250 mg (group 2), and fourteen patients receiving 500 mg (group 3) of furosemide. Urine samples of all patients were collected at 30, 60, and 90 min, and 3, 4, 5, 6, 8, and 24 h after infusion. Diuresis, sodium excretion, osmolality, and furosemide concentration were evaluated for each urine sample.
Results
After randomization, 40 patients completed the study. Two patients, one in group 2 and one in group 3 dropped out. Patients in group 1 (125 mg furosemide) had a mean age of 77 ± 17 years, 43% were male, 6 (43%) had heart failure with a preserved ejection fraction (HFpEF), and 64% were in New York Heart Association (NYHA) class IV; the mean age of patients in group 2 (250 mg furosemide) was 80 ± 8.1 years, 15% were male, 5 (38%) had HFpEF, and 84% were in NYHA class IV; and the mean age of patients in group 3 (500 mg furosemide) was 73 ± 12 years, 54% were male, 6 (46%) had HFpEF, and 69% were in NYHA class IV. HSS added to furosemide increased total urine output, sodium excretion, urinary osmolality, and furosemide urine delivery in all patients and at all time points. The percentage increase was 18,14, and 14% for urine output; 29, 24, and 16% for total sodium excretion; 45, 34, and 20% for urinary osmolarity; and 27, 36, and 32% for total furosemide excretion in groups 1, 2, and 3, respectively. These findings were translated in an improvement in the furosemide dose–response curves in these patients.
Conclusion
These results may serve as new pathophysiological basis for HSS use in the treatment of refractory CHF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is characterized by progressive fluid accumulation and a gradually decreased response to diuretic therapy [1]. Although the overall impact of diuretic therapy on HF mortality remains unclear, diuretics remain a mainstay of HF therapy [2, 3]. However, chronic treatment with diuretics may result in a phenomenon called “chronic braking” which limits the diuretic response and deteriorates the clinical status. When the sodium and water excretion rate plateaus are achieved before adequate fluid elimination, a condition known as diuretic resistance occurs [4]. Pharmacokinetic and pharmacodynamic alterations are thought to be responsible for diuretic resistance in patients with HF [5]. As a matter of fact, the chronic use of loop diuretics leads to a functional adaptation of the distal tubule that alters its ability for reabsorption and results in diuretic resistance [6]. Sodium reabsorption in the distal tubule increases significantly when loop diuretics augment sodium delivery to this segment.
Brater et al. [7] described the response to intravenous (IV) furosemide administration using a fit sigmoid-shaped curve in patients with HF and in healthy controls evidencing that in HF, the curve shifts down and to the right indicating reduced furosemide and sodium excretion into urine. Moreover, several studies in patients with chronic HF (CHF) have reported that IV infusion of hypertonic saline solution (HSS) plus high-dose furosemide is more effective than furosemide alone [8–12], resulting in increased urine output. These findings have allowed for greater weight loss, a shorter length of hospitalization [13–16], a greater reduction in neurohormonal activation [17], and a significant improvement in renal function [18–21].
To validate the promising effects of HSS administration, the primary endpoint of the present study was to investigate the behavior on the dose–response curve after IV administration of high doses of furosemide diluted in HSS in comparison with the same dose of furosemide diluted in normal saline in patients hospitalized with refractory CHF.
Methods
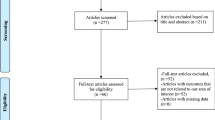
Patients were suitable for recruitment if they had presented with acute decompensated HF in the previous 24 h; diagnosis was made on the basis of the manifestation of at least one symptom (dyspnea, orthopnea, or edema) and one sign (pulmonary rales, lower limb edema, ascites, or lung vascular congestion on chest radiography) of HF according to European Society of Cardiology Criteria [22]. Additional inclusion criteria were a history of CHF treated with oral loop diuretics, at a dose of between 125 mg and 500 mg daily of furosemide, for at least 1 month before hospitalization. There were no pre-specified inclusion criteria regarding left ventricular ejection fraction (LVEF). Patients with 90 mm Hg or less of systolic blood pressure (BP) or with a serum creatinine level >2.5 mg/dL (265.2 μmol/L) were excluded. In addition, patients who needed vasodilators or inotropic agents via IV (other than digoxin) or non-steroidal anti-inflammatory drugs were also excluded. Protocol was carried out following the principles of the Helsinki Declaration of 1964, and subsequent revisions, and in accordance with national legislations. The hospital’s Internal Review Board and its Ethics Committee approved the protocol and all patients signed a written informed consent (Fig. 1).
The study involved 42 consecutively hospitalized patients with refractory CHF fulfilling all of the eligibility criteria. Patients were randomly assigned in a 1:1:1 ratio to furosemide doses (125 mg, 250 mg, 500 mg) so that all patients received both IV infusions over 20 min every 24 h, the first of which contained furosemide diluted in 150 mL of normal saline (0.9%) and the last the same furosemide dose diluted in 150 mL of HSS (1.4%). Randomization was performed with the use of a preliminary computer algorithm, and a complete clinical examination and laboratory measurements were carried out before it.
An independent team of nurses prepared the solutions and had an independent physician follow the process. A complete physical examination, including body weight (BW), BP, and heart rate (HR), was performed in all selected patients after randomization. Serum Na, K, Cl, bicarbonate, albumin, uric acid, creatinine, blood urea nitrogen (BUN) and glucose were determined through venous blood samples before treatment. Just after IV bolus of furosemide, urine samples were collected and measured using an urinometer, according to the protocol described by Brater et al. [7], at 30, 60, and 90 min, and at 3, 4, 5, 6, 8, and at 24 h (T1–T9) after normal saline and HSS. This was given in association with fluid intake restriction (1000 mL/day) and a dietary sodium intake of 2.8 g/day (120 mmol/day). Urine samples were consecutively numbered and signed by physicians blinded to the study protocol, and immediately frozen at −20 °C. Samples were subsequently delivered to the Regional Quality Control Laboratory (RQCL) for the quantification of urinary furosemide, natriuresis, and osmolality and analyzed by an external blinded team of physicians. Urinary excretion of furosemide was assayed with HPLC–MS/MS (High-Performance Liquid Chromatography–tandem mass spectrometry) method [13]. Urine furosemide excretion data was processed by an external computer scientist, blinded to the study protocol, to obtain dose–response curves.
Echocardiographic Analysis
Echocardiograms using a GE Vivid™ 7 Dimension (GE Healthcare) ultrasonography were detected at entry and 48 h later at the end of the protocol. Standard parasternal and apical views images were acquired with a phased-array transducer in the left lateral decubitus position. The average of three consecutive cycles was calculated to determine all echocardiographic measurements. LV volumes, LVEF, mitral regurgitation, and left atrial maximum volume were assessed according to the American Society of Echocardiography criteria [23].
Furosemide Determination
Standard solutions of furosemide and hydrochlorothiazide (HCT; purities ≥98% and ≥99%, respectively) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Methanol (purity LC–MS) was obtained from JT Baker® (Avantor Performance Materials, Inc., Center Valley, PA, USA). Formic acid (purity approximately 98%) was obtained from Sigma-Aldrich. The water used, HPLC-grade, was obtained from distilled water for purification, via the purification system Barnstead Nanopure Diamond (Thermo Fisher Scientific, Palo Alto, CA, USA). Stock solutions of furosemide and HCT (used as internal standard) were obtained by dissolving 15.0 mg of standard solids, accurately weighed, in 100 mL of methanol, obtaining solutions of 150 mg L−1 (150 ppm) concentration, which were stored at −20 °C. Working solutions used for the analysis in LC–MS/MS or for the creation of urine samples fortified were obtained starting from stock solutions by dilution with water or methanol, using a dilution factor less than 10 in each step of dilution.
HPLC–MS/MS Analysis [24]
LC–MS/MS analyses were performed using an HPLC Accela 1000 (Thermo Fisher Scientific) equipped with a refrigerated autosampler, degasser, and thermostatic chamber for the column chromatographic, interfaced to a tandem-mass high-resolution spectrometer Q-Exactive (Thermo Fisher Scientific) coupled with atmospheric pressure heated source, H-ESI II. Chromatographic separation was obtained using a Thermo HyperSil Gold C18 PFP column (50 mm × 2.1 mm i.d., 1.9 μm particle size), thermostated at 25 °C. The chromatographic run was performed using the method on gradient concentration of two eluents: A (water + 0.1% formic acid) and B (methanol + 0.1% formic acid). 100 μL of centrifuge urine, placed in a 1-mL volumetric flask, was added with 10 μL of HCT solution (concentration 50 mg L−1) and was brought to the final volume with HPLC-water. 5 μL of the resulting solutions were directly injected into the chromatographic system. Quantification of the samples was carried out using calibration curves of furosemide in matrix, according to the internal standard method, in the range between 0.025 and 15 mg L−1. The fortified urine samples used for the calibration curves were obtained by adding to 100 μL of centrifuged blank urine, appropriate volumes of working solution of furosemide and a fixed volume of 10 μL of HCT (50 mg L−1) and brought to the final volume of 1 mL, with HPLC-grade water. Chromatograms obtained were processed using the quantification tool Quan Browser Xcalibur® (Thermo Electron Corporation). Calibration curves were obtained by plotting the relationship between the values chromatographic areas—obtained by extrapolating the track in full scan signal relative to the ion of mass number/charge number (m/z) 328.9993 (furosemide) and m/z 295.9572 (HCT) (with a tolerance of 5 ppm on the reading value of m/z)—versus the concentration of furosemide. At the beginning and end of each run, two calibration curves were inserted and the straight curves obtained reported R2 values P > 0.9978.
Data Analysis
Computer fitting to a sigmoid-shaped curve of furosemide excretion rate and response “dose–response curves” relationship was examined using the following formula:
where Y (response) is the urinary sodium excretion rate, X is the furosemide excretion rate, a is the lower asymptote (i.e., response when dose = 0), b is the “slope factor” that determines inclination of the curve; c is the dose representing half-maximal response (d − a)/2, and d shows the upper asymptote (i.e., response when dose = ∞). Individual data were fit and compared using the ALLFIT computer program [25]. For each curve, the software assigns a P value, which represents an index of representativeness of the curve compared to experimental data: good (P > 0.05), poor (P < 0.05), and bad (P < 0.01), as indication of the quality of the curve fit.
Results
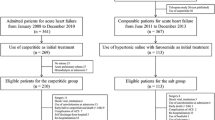
After randomization, the three groups of patients who received one of the IV diuretic dosages were: fourteen patients who received 125 mg of furosemide (group 1), thirteen patients who received 250 mg of furosemide (group 2), and thirteen patients received 500 mg of furosemide (group 3). One patient in group 2 and one patient in group 3 dropped out because they did not tolerate the bladder catheterization and were therefore not analyzed. A comparison of the baseline features of the cohorts of randomized patients is shown in Table 1. The mean age of the patients in group 1 (125 mg) was 77 ± 17 years, 43% were male, 6 (43%) had heart failure with a preserved ejection fraction (HFpEF), and 64% were in New York Heart Association (NYHA) class IV; the mean age of patients in group 2 (250 mg) was 80 ± 8.1 years, 15% were male, 5 (38%) had HFpEF, and 84% were in NYHA class IV; the mean age of patients in group 3 (500 mg) was 73 ± 12 years, 54% were male, 6 (46%) had HFpEF, and 69% were in NYHA class IV. There were no substantial differences between the groups, neither in CHF etiology nor in the different types of medical therapy. All patients were receiving treatment with furosemide orally, overall 45% had severe lower limb edema, and 43% had a reduced LVEF. No significant differences in serum sodium, creatinine, blood urea nitrogen (BUN), systolic blood pressure (BP), and heart rate (HR) were found between the groups (Table 2). Total furosemide excretion, urinary osmolarity, urinary sodium, and 24-h diuresis in the study population after furosemide plus HSS administration in comparison with furosemide plus normal saline baseline are presented in Fig. 2. The addition of HSS to furosemide dose improved total diuresis, urine osmolarity, furosemide, and sodium excretion in all groups. There was an increase in the excretion of furosemide of between 27% and 36% and in the total sodium excretion of between 16% and 29% when HSS was added. An increase in 24-h urine output of between 14% and 18% compared to baseline was also observed. Similar behavior was observed for total urinary osmolality (Table 3). The link between furosemide release and natriuretic response is shown in Fig. 3. Each point represents the median of one urine collection period and the curve encompassed the time course of the entire study (24 h). In approximately 85% of curves, the software assigned a good P value (P > 0.05) indicating the quality of the curve. In all groups, the median of furosemide plus HSS shows a dose–response curve up and to the left while the median dose response curve obtained with furosemide plus normal saline is down and on the right.
Relationship between the delivery of furosemide and natriuretic response in patients treated with furosemide and furosemide plus HSS. Curves show the behavior of each patient in response to the two treatments. a Median of 14 patients treated with 125 mg furosemide (whole line) and 125 mg furosemide plus HSS (dashed line). b Median of 13 patients treated with 250 mg furosemide (whole line) and 250 mg plus HSS (dashed line). c Median of 13 patients treated with 500 mg furosemide (whole line) and 500 mg furosemide plus HSS (dashed line). Fu furosemide, HSS hypertonic saline solution
Discussion
The amount of drug reaching the renal tubule is the most important determinant of the response to a diuretic [26]. The drug can be quantified by the ratio of sodium excretion relative to urinary diuretic concentration [27]. Glomerular filtration rates are relatively normal in most patients with CHF but their renal blood flow is reduced [28]. Since furosemide gains access to its intraluminal site of action by active secretion at the proximal tubule, a decreased blood flow could limit delivery to the secretory site. Consequently, patients with CHF may not respond because of reduced delivery of diuretic to the site of action. The present study suggests that furosemide tubular delivery increases over time in all groups but a higher furosemide concentration is observed when HSS is added. The relationship between excretion of sodium and urine furosemide delivery was represented as a curve encompassing the time course of the entire study, fitted as a sigmoid function. To explain these data, we assumed that the effect of the infusion of HSS has realized a dual action that would justify the observed variation of the excretion pattern of sodium and furosemide, and, therefore, the shift of the dose–response curve at the top and to the left, with a closer-to-physiology pattern. The shift at the top of the curve intuitively reflects the increased urinary sodium observed in the whole study population after HSS infusion. Moreover, the observation that urinary osmolarity, urinary sodium, and 24-h diuresis were higher at all dosages of diuretic administration when HSS was added is the consequence of major furosemide and sodium excretion. Such consequences were probably due to the favorable diuretic effects of HSS that modified pharmacokinetics and pharmacodynamics of furosemide. In fact, due to its osmotic effect, HSS causes a fast and instantaneous mobilization of fluids from the third space to the vascular compartment, without a significant simultaneous rising of serum sodium. HSS exerts its positive effects in the kidney acting as a sort of “bait” for the action of the diuretic by facilitating its action with a twofold effect [29, 30]. Intuitively, these results are reliable with an increase in effective kidney blood volume that leads to an explanation for the quantitative and qualitative variations of urine. Another explanation may consist in the functional attitude of the kidney that changes in response to a load of sodium and water. Deep nephrons, due to their well-developed loops of Henle, are more efficient in the reabsorption of water and sodium (salt-saving nephrons) while superficial nephrons due to their short developed loops of Henle are more efficient in the excretion of water and sodium (salt-losing nephrons) [31]. During increased loads of water and sodium occurring after administration of HSS, there is a change in the auto regulation of renal plasma flow, more marked in the cortex rather than in the renal medulla, and, as a consequence, blood flow is increased and the filtration rate in the superficial nephrons (salt-losing nephrons) is also increased [31].
The present investigation shows that the beneficial effects of HSS evidenced in previous studies [8–12, 15, 16] are supported by the fact that HSS administered in conjunction with furosemide improves diuretic and sodium excretion rate, and subsequently shifts the furosemide dose–response curves up and to the left. These data suggest that this approach is effective, and may be adopted into routine care and should serve as a new pathophysiological basis for the management of decompensated refractory CHF. Moreover, these data, if confirmed, add new insights in understanding mechanisms of diuretic administration in patients with CHF.
References
Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119(12 suppl 1):S3–10.
Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–53.
Valente MAE, Voors AA, Damman K, et al. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J. 2014;35(19):1284–93.
Kramer BK, Schweda F, Riegger GA. Diuretic treatment and diuretic resistance in heart failure. Am J Med. 1999;106:90–6.
Brater DC. Pharmacokinetics of loop diuretics in congestive heart failure. Br Heart J. 1994;72(Suppl):S40–3.
Dormans T, Gerlag P, Russel F, et al. Combination diuretic therapy in severe congestive heart failure. Drugs. 1998;55:165–72.
Brater DC, Chennasasin P, Seiwell R. Furosemide in patients with heart failure. Shift of the dose response relationship. Clin Pharmacol Ther. 1980;28:182–6.
Paterna S, Parrinello G, Amato P, et al. Tolerability and efficacy of high-dose furosemide and small 17 volume hypertonic saline solution in refractory congestive heart failure. Adv Ther. 1999;16:219–28.
Paterna S, Parrinello G, Amato P, et al. Small volume hypertonic saline solution and high-dosage furosemide in the treatment of refractory congestive heart failure. A pilot study. Clin Drug Invest. 2000;19:9–13.
Paterna S, Di Pasquale P, Parrinello G, et al. Effects of high-dose furosemide and small volume hypertonic saline solution infusion in comparison with a high dose of furosemide as a bolus, in refractory congestive heart failure. Eur J Heart Fail. 2000;2:305–13.
Licata G, Di Pasquale P, Parrinello G, et al. Effects of high-dose furosemide and small-volume hypertonic saline solution in comparison with a high dose of furosemide as bolus in refractory congestive heart failure: long term effects. Am Heart J. 2003;145:459–66.
Paterna S, Di Pasquale P, Parrinello G, et al. Changes in brain natriuretic peptide levels and bioelectrical impedance measurements after treatment with high-dose furosemide and hypertonic saline solution versus high-dose furosemide alone in refractory congestive heart failure. J Am Coll Cardiol. 2005;45:1997–2003.
Gandhi S, Mosleh W, Myers RB. Hypertonic saline with furosemide for the treatment of acute congestive heart failure: a systematic review and meta-analysis. Int J Cardiol. 2014;173(2):139–45.
Ventrella F, Cappello S, Minafra G, et al. Hypertonic saline solution and high-dose furosemide infusion in cardiorenal syndrome: our experience. Ital J Med. 2012;6:91–8.
Tuttolomondo A, Pinto A, Parrinello G, Licata G. Intravenous high-dose furosemide and hypertonic saline solutions for refractory heart failure and ascites. Semin Nephrol. 2011;31(6):513–22.
Tuttolomondo A, Pinto A, Di Raimondo D, et al. Changes in natriuretic peptide and cytokine plasma levels in patients with heart failure, after treatment with high dose of furosemide plus hypertonic saline solution (HSS) and after a saline loading. Nutr Metab Cardiovasc Dis. 2011;21(5):372–9.
Miller WL, Borgeson DD, Grantham JA, Luchner A, Redfield MM, Burnett JC Jr. Dietary sodium modulation of aldosterone activation and renal function during the progression of experimental heart failure. Eur J Heart Fail. 2015;17(2):144–50.
De Vecchis R, Ciccarelli A, Ariano C, et al. Renoprotective effect of small volumes of hypertonic saline solution in chronic heart failure patients with marked fluid retention: results of a case-control study. Herz. 2011;36(1):12–7.
Issa VS, Andrade L, Ayub-Ferreira SM, et al. Hypertonic saline solution for prevention of renal dysfunction in patients with decompensated heart failure. Int J Cardiol. 2013;167(1):34–44.
De Vecchis R, Esposito C, Ariano C, Cantatrione S. Hypertonic saline plus i.v. furosemide improve renal safety profile and clinical outcomes in acute decompensated heart failure: a meta-analysis of the literature. Herz. 2014;39(1):1–15.
Issa VS, Bacal F, Mangini S, et al. Hypertonic saline solution for renal failure prevention in patients with decompensated heart failure. Arq Bras Cardiol. 2007;89(4):251–5.
McMurray JV, Adamopoulos S, Anker S, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012;33:1787–847.
Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography: American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358e67.
Sora DI, Udrescu S, Albu F, et al. Analytical issues in HPLC/MS/MS simultaneous assay of furosemide, spironolactone and canrenone in human plasma samples. J Pharm Biomed Anal. 2010;52:734–40.
DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay and physiological dose-response curves. Am J Physiol Endocrinol Metab. 1978;235:E97–102.
Brater DC, Chennavasin P, Day B, et al. Bumetanide and frusemide. Clin Pharmacol Ther. 1983;34:207–13.
Benet LZ. Pharmacokinetics/pharmacodynamics of frusemide in man: a review. J Pharmacokinet Biopharm. 1979;7:1–27.
Cannon PJ. The kidney in heart failure. N Engl J Med. 1977;296:26–32.
Gabrielsen A, Bie P, Holstein-Rathlou NH, et al. Neuroendocrine and renal effects of intravascular volume expansion in compensated heart failure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R459–67.
Damgaard M, Norsk P, Gustafsson F, et al. Hemodynamic and neuroendocrine responses to changes in sodium intake in compensated heart failure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1294e301.
Regel JA. Comparative physiology of renal excretion. Edinburgh: Oliver & Boyd; 1972.
Acknowledgments
No funding was received for this study. The article processing charges and the open access fee were funded by the authors. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Salvatore Paterna, Francesca Di Gaudio, Vincenzo La Rocca, Fabio Balistreri, Massimiliano Greco, Daniele Torres, Umberto Lupo, Giuseppina Rizzo, Pietro di Pasquale, Sergio Indelicato, Francesco Cuttitta, Javed Butler, and Gaspare Parrinello have no conflict of interest to declare and no relationship with industry.
Compliance with ethics guidelines
Protocol was carried out following the principles of the Helsinki Declaration of 1964, and subsequent revisions, and in accordance with national legislations. The hospital’s Internal Review Board and its Ethics Committee approved the protocol and all patients signed a written informed consent.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Paterna, S., Di Gaudio, F., La Rocca, V. et al. Hypertonic Saline in Conjunction with High-Dose Furosemide Improves Dose–Response Curves in Worsening Refractory Congestive Heart Failure. Adv Ther 32, 971–982 (2015). https://doi.org/10.1007/s12325-015-0254-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-015-0254-9