Abstract
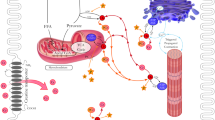
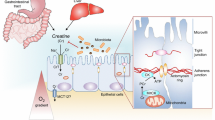
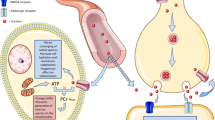
Since the 1970s, extensive experimental and clinical research has demonstrated that relevant reductions of creatine phosphate (CrP) or phosphocreatine availability occur in a wide spectrum of pathophysiological situations. A decrease in intracellular concentrations of creatine (Cr) and CrP results in a hypodynamic state of cardiac and skeletal muscle pathology. Many experimental and clinical studies have evaluated the possibility to improve cardiac and skeletal muscle performance by exogenous administration of CrP. Furthermore, many experimental studies have shown that CrP may play two important roles in the regulation of muscle energetics and work. First, CrP maintains local adenosine triphosphate pools and stabilizes cellular membranes due to electrostatic interactions with phospholipids. The second mechanism decreases the production of lysophosphoglycerides in hypoxic hearts, protects the sarcolemma of cardiac cells against ischemic damage, decreases the frequency of arrhythmias, and increases post-ischemic recovery of contractile function. Recent research on CrP has demonstrated positive therapeutic results in various clinical applications. These benefits have been applied in several pathological conditions, such as heart failure, acute myocardial ischemia, chronic ischemic heart disease, cardiac surgery, skeletal muscle hypotonotrophy, and cerebral ischemia. This review describes the CrP shuttle, pathophysiological basis of the supplementation of CrP, and its therapeutic effects in multiple clinical conditions. The major aim is to summarize results of the intense research carried out over 40 years to provide evidence to support the adjunctive use of CrP in many pathological conditions that may target cellular energy impairment; thus, increasing energy metabolism.
Similar content being viewed by others
References
Walker JB. Creatine: biosynthesis, regulation, and function. Adv Enzymol Relat Areas Mol Biol. 1979;50:117–242.
Ennor AH, Morrison JF. Biochemistry of the phosphagens and related guanidines. Physiol Rev. 1958;38:631–674.
Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211:448–452.
Bessman SP, Mohan C. Phosphocreatine, exercise, protein synthesis, and insulin. In: PP De Deyn, B. Marescau, V. Stalon an IA Qureshi, eds. Guanidino Compounds in Biology and Medicine. London: John Libbey and Company; 1992:181–186.
Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145.
Taegtmeyer H, Wilson CR, Razeghi P, Sharma S. Metabolic energetics and genetics in the heart. Ann N Y Acad Sci. 2005;1047:208–218.
Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419.
Saks VA, Ventura-Clapier R, Huchua ZA, Preobrazhensky AN, Emelin IV. A creatine kinase in regulation of heart function and metabolism. I. Further evidence for compartmentation of adenine nucleotides in cardiac myofibrillar and sarcolemmal coupled ATPase-creatine kinase systems. Biochim Biophys Acta. 1984;803:254–264.
Carpenter CL, Mohan C, Bessman SP. Inhibition of protein and lipid synthesis in muscle by 2,4-dinitrofluorobenzene, an inhibitor of creatine phosphokinase. Biochem Biophys Res Commun. 1983;111:884–889.
Hearse DJ. Oxygen deprivation and early myocardial contractile failure: a reassessment of the possible role of adenosine triphosphate. Am J Cardiol. 1979;44:1115–1121.
Whitman GJ, Kieval RS, Seeholzer S, et al. Recovery of left ventricular function after graded cardiac ischemia as predicted by myocardial P-31 nuclear magnetic resonance. Surgery. 1985;97:428–435.
Pool PE, Spann JF Jr, Buccino RA, Sonnenblick EH, Braunwald E. Myocardial high energy phosphate stores in cardiac hypertrophy and heart failure. Circ Res. 1967;21:365–375.
Ye Y, Gong G, Ochiai K, Liu J, Zhang J. High-energy phosphate metabolism and creatine kinase in failing hearts: a new porcine model. Circulation. 2001;103:1570–1576.
Weiss RG, Bottomley PA, Hardy CJ, Gerstenblith G. Regional myocardial metabolism of high energy phosphates during isometric exercise in patients with coronary artery disease. N Engl J Med. 1990;323:1593–1600.
Yabe T, Mitsunami K, Okada M, et al. Detection of myocardial ischemia by 31P magnetic resonance spectroscopy during handgrip exercise. Circulation. 1994;89:1709–1716.
Yabe T, Mitsunami K, Inubushi T, Kinoshita M. Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31P magnetic resonance spectroscopy. Circulation. 1995;92:15–23.
Conway MA, Allis J, Ouwerkerk R, et al. Detection of low creatine phosphate to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991;338:973–976.
Hardy CJ, Weiss RG, Bottomley PA, Gerstenblith G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am Heart J. 1991;122:795–801.
Neubauer S, Krahe T, Schindler R, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818.
Naveri HK, Leinonen H, Kiilavuori K, Härkönen M. Skeletal muscle lactate accumulation and creatine phosphate depletion during heavy exercise in congestive heart failure. Cause of limited exercise capacity? Eur Heart J. 1997;18:1937–1945
Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196.
Chida K, Otani H, Kohzuki M, et al. The relationship between plasma BNP level and the myocardial phosphocreatine/adenosine triphosphate ratio determined by phosphorus-31 magnetic resonance spectroscopy in patients with dilated cardiomyopathy. Cardiology. 2006;106:132–136.
Starling RC, Hammer DF, Altschuld RA. Human myocardial ATP content and in vivo contractile function. Mol Cell Biochem. 1998;180:171–177.
Nascimben L, Ingwall JS, Pauletto P, et al. Creatine kinase system in failing and nonfailing human myocardium. Circulation. 1996;94:1894–1901.
Tian R, Ingwall JS. The molecular energetics of the failing heart from animal models-small animal models. Heart Failure Rev. 1999;4:235–253.
Zhang J, Bache RJ. The molecular energetics of the failing heart from animal models-large animal models. Heart Failure Rev. 1999;4:255–267.
Ingwall JS, Kramer MF, Fifer MA, et al. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985;313:1050–1054.
Katz AM. Is the failing heart energy depleted? Cardiol Clin. 1998;16:633–644, viii.
Nakae I, Mitsunami K, Omura T, et al. Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. J Am Coll Cardiol. 2003;42:1587–1593.
van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res. 2004;61:218–226.
Neubauer S. The failing heart-an engine out of fuel. N Engl J Med. 2007;356:1140–1151.
Editorial: Skeletal muscle in heart failure. Lancet. 1992;340:1383–1384.
Drexler H, Münzel T, Riede U, Just H. Adaptive changes in the periphery and their therapeutic consequences. Am J Cardiol. 1991;67:29C–34C; discussion 34C–35C.
Drexler H. Skeletal muscle failure in heart failure. Circulation. 1992;85:1364–1373.
Drexler H, Coats AJ. Explaining fatigue in congestive heart failure. Annu Rev Med. 1996;47:241–256.
Massie BM, Conway M, Rajagopalan B, et al. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation. 1988;78:320–326.
Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373.
Mancini DM, Wilson JR, Bolinger L, et al. In vivo magnetic resonance spectroscopy measurement of deoxymyoglobin during exercise in patients with heart failure. Demonstration of abnormal muscle metabolism despite adequate oxygenation. Circulation. 1994;90:500–508.
Lunde PK, Sjaastad I, Schiøtz Thorud HM, Sejersted OM. Skeletal muscle disorders in heart failure. Acta Physiol Scand. 2001;171:277–294.
Parratt JR, Marshall RJ. The response of isolated cardiac muscle to acute anoxia: protective effect of adenosine triphosphate and creatine phosphate. J Pharm Pharmacol. 1974;26:427–433.
Marshall RJ, Parratt JR. Reduction in ventricular arrhythmias following acute coronary artery ligation in the dog after the administration of creatine phosphate. Naunyn Schmiedeberg’s Arch Pharmacol. 1974;281:437–441.
Fagbemi O, Kane KA, Parratt JR. Creatine phosphate suppresses ventricular arrhythmias resulting from coronary artery ligation. J Cardiovasc Pharmacol. 1982;4:53–58.
Rosenshtraukh LV, Saks VA, Anyukhovsky EP, Beloshapko GG, Yushmanova AV. The antiarrhythmic action of creatine phosphate in acute myocardial ischemia. Biochem Med. 1985;34:120–128.
Hearse DJ, Tanaka K, Crome R, Manning AS. Creatine phosphate and protection against reperfusion-induced arrhythmias in the rat heart. Eur J Pharmacol. 1986;131:21–30.
Sharov VG, Afonskaya NI, Ruda MY, et al. Protection of ischemic myocardium by exogenous phosphocreatine: Pharmacokinetics of phosphocreatine, reduction of infarct size, stabilization of sarcolemma of ischemic cardiomyocytes and antithrombotic action. Biochem Med Metab Biol. 1986;35:101–114.
Woo YJ, Grand TJ, Zentko S, et al. Creatine phosphate administration preserves myocardial function in a model of off-pump coronary revascularization. J Cardiovasc Surg. 2005;46:297–305.
Robinson LA, Braimbridge MV, Hearse DJ. Creatine phosphate: an additive myocardial protective and antiarrhythmic agent in cardioplegia. J Thorac Cardiovasc Surg. 1984;87:190–200.
Sharov VG, Saks VA, Kupriyanov VV, et al. Protection of ischemic myocardium by exogenous creatine phosphate: morphologic and phosphorus 31-nuclear magnetic resonance studies. J Thorac Cardiovasc Surg. 1987;94:749–761.
Thelin S, Hultman J, Ronquist G, et al. Improved myocardial protection by creatine phosphate in cardioplegic solution. An in vivo study in the pig during normothermic ischemia. Thorac Cardiovasc Surg. 1987;35:137–142.
Down WH, Chasseaud LF, Ballard SA. The effect of intravenously administered phosphocreatine on ATP and phosphocreatine concentrations in the cardiac muscle of the rat. Arzneimittelforschung. 1983;33:552–554.
Breccia A, Fini A, Girotti S, Gattavecchia E. Intracellular distribution of double-labelled creatine phosphate in the rabbit myocardium. Curr Ther Res. 1985;37:1205–1215.
Preobrazhensky AN, Javadov SA, Saks VA. Study of the hypothetical mechanism of protective effect of phosphocreatine on ischemic myocardium. Biochimia. 1986;51:675–683.
Jacobus WE. Respiratory control and the integration of heart high-energy phosphate metabolism by mithocondrial creatine kinase. Annu Rev Physiol. 1985;47:707–725.
Saks VA, Strumia E. Phosphocreatine: Molecular and cellular aspects of the mechanism of cardioprotective action. Curr Ther Res. 1993;53:565–598.
Ronca-Testoni A, Raggi A, Ronca G. Muscle AMP aminohydrolase. Comparative study on the regulatory properties of skeletal muscle enzyme from various species. Biochim Biophys Acta. 1970;198:101–112.
Saks VA, Javadov SA, Pozin E, Preobrazhensky AN. Biochemical basis of the protective action of phosphocreatine on the ischemic myocardium. In: Saks VA, Bobkov, Strumia E, eds. Proceedings of the Symposium “Creatine phosphate-biochemistry, pharmacology and clinical efficiency” Baku, October 1986. Torino: Edizioni Minerva Medica; 1987:95–111.
Rubio R, Belardinelli L, Thompson CI, Berne RM. Cardiac adenosine: Electrophysiological effects, possible significance in cell function and mechanism controlling its release. In: Baer HP, Drummond GI, eds. Physiological and Regulatory Functions of Adenosine and Adenine Nucleotides. New York: Raven Press; 1979:167–183.
Ronca G, Ronca-Testoni S, Conte A, et al. Creatine phosphate and protection from peroxidative damage. Proceedings of the International Meeting “Cardioprotection with phosphocreatine in cardiology and cardiac surgery” IRCCS, Policlinical S. Matteo, Università degli Studi, Pavia, Italy, April 1989:61–71.
Zucchi R, Poddighe R, Limbruno U, et al. Protection of isolated rat heart from oxidative stress by exogenous phosphocreatine. J Mol Cell Cardiol. 1989;21:67–73.
Konorev EA, Sharov VG, Saks VA. Improvement in contractile recovery of isolated rat heart after cardioplegic ischemic arrest with endogenous phosphocreatine: Involvement of antiperoxidative effect. Cardiovasc Res. 1991;25:164–171.
Corr PB, Witkowski FX, Sobel BE. Mechanism contributing to malignant dysrhythmias induced by ischemia in the cat. J Clin Invest. 1978;61:109–119.
Sobel BE, Corr PB. Accumulation of lysophosphoglycerides with arrhythmogenic properties in ischemic myocardium. J Clin Invest. 1978;62:546–553.
Saks VA, Kapelko VI, Ruda MY, Semenovski ML, Strumia E. Phosphocreatine as effective drug in clinical cardiology. In: De Deyn PP, Marescau B, Stalon V, Qureshi IA, eds. Guanidino Compounds in Biology and Medicine. London: John Libbey & Company; 1992:239–248.
Guzun R, Timohhina N, Tepp K, et al. Systems bioenergetics of creatine kinase networks: physiological roles of creatine and phosphocreatine in regulation of cardiac cell function. Amino Acids. 2011;40:1333–1348.
Hearse DJ, Brainbridge MV, Jynge P. Protection of the Ischemic Myocardium: Cardioplegia. New York: Raven Press; 1981:151–156.
Semenovsky ML, Shumakov VI, Sharov VG, et al. Protection of ischemic myocardium by exogenous creatine phosphate. II. Clinical, ultrastructural and biochemical evaluations. J Thorac Cardiovasc Surg. 1987;94:762–769.
D’Alessandro LC, Cini R, Pucci A, et al. Protezione miocardica: uso del creatin fosfato addizionato alla soluzione cardioplegica. Heart Surgery 1987, 2nd International Symposium on Cardiac Surgery, Roma 1987, Casa Editrice Internazionale;179–192.
Chambers DJ, Braimbridge MV, Kosker S, et al. Creatine phosphate (Neoton) as an additive to St. Thomas’ Hospital cardioplegic solution (Plegisol). Results of a clinical study. Eur J Cardiothor Surg. 1991;5:74–81.
Chambers DJ, Haire K, Morley N, et al. St. Thomas’ Hospital cardioplegia: enhanced protection with exogenous creatine phosphate. Ann Thor Surg. 1996;61:67–75.
Mastroroberto P, Di Tommaso L, Chello M, et al. Creatine phosphate protection of the ischemic myocardium during cardiac surgery. Curr Ther Res. 1992;51:37–45.
Pauletto P, Nascimben L, De Ros S, et al. Prevention of arrhythmias and changes in myocardial enzyme release with creatine phosphate in patients undergoing coronary artery by-pass. J Mol Cell Cardiol. 1993;25(Suppl. 3):109 (Abstract).
Pastoris O, Dossena M, Vercesi L, et al. Biochemical changes induced in the myocardial cell during cardioplegic arrest supplemented with creatine phosphate. J Cardiothorac Vasc Anesth. 1991;5:475–480.
Pagani L, Musiani A. The use of systemic phosphocreatine in heart surgery. Minerva Anestesiol. 1992;58:199–205.
Cisowski M, Bochenek A, Kucewicz E, et al. The use of exogenous creatine phosphate for myocardial protection in patients undergoing coronary artery bypass surgery. A clinical assessment. J Cardiovasc Surg. 1996;37:75–80.
Thorelius J, Thelin S, Ronquist G, Halden E, Hansson HE. Biochemical and functional effects of creatine phosphate in cardioplegic solution during aortic valve surgery. A clinical study. Thorac Cardiovasc Surg. 1992;40:10–13.
Cossolini M, Sonzogni V, Di Dedda G, et al. Paediatric cold heart surgery: experience with creatine phosphate added to cardioplegic solution. In: D’Alessandro LC, ed. Heart Surgery. Roma: Casa Editrice Scientifica Internazionale; 1993:442–443.
Ruda MY, Samarenko MB, Afonskaya NI, Saks VA. Reduction of ventricular arrhythmias by creatine phosphate in patients with acute myocardial infarction. Am Heart J. 1988;116:393–397.
Reimers B, Maddalena F, Cacciavilani L, et al. La fosfocreatina nell’infarto miocardico acuto: studio randomizzato multicentrico. Il Cuore. 1994;11:345–354.
Scattolin G, Gambino A, Bellotto F, et al. Phosphocreatine in acute myocardial infarction. In: Saks VA, Bobkov YG, Strumia E, eds. Proceeding of the Symposium “Creatine Phosphate: Biochemistry, Pharmacology and Clinical Efficiency”, Baku, 6 October 1986. Torino: Minerva Medica; 1987;199–205.
Coraggio F, Spina M, Scarpato P. Analysis of creatine phosphate on the evolution of ischemic lesion in acute myocardial infarction. Farmaci Terapia. 1987;4:91–93.
Cini R, Stazi G, Giacopino F, et al. Creatinfosfato nella fase acuta dell’infarto miocardico. Risultati preliminari di uno studio clinico. Il Cuore. 1987;4:91–102.
Raisaro A, Bargiggia CS, Bertucci C, et al. Clinical evaluation of phosphocreatine effect during acute myocardial infarction: A multicenter study. Proceedings of the International Meeting “Cardioprotection with phosphocreatine in cardiology and cardiac surgery” IRCCS, Policlinico S. Matteo, Università degli studi, Pavia; 1989:139–147.
Camilova UK, Katsenovich RA, Kostco SZ. Combined use of creatine phosphate and nifedipine for treatment of patients with acute myocardial infarction. Curr Ther Res. 1991;50:591–598.
Iosseliani DG, Koledinsky AG, Kuchkina NV. Does intracoronary injection of phosphocreatine prevent myocardial reperfusion injury following angioplasty of infarct-related artery in acute-stage of myocardial infarction? J Intervent Cardiol. 2004;6:10–14.
Iosseliani DG, Koledinsky AG, Kuchkina NV. The possibility to limit reperfusion injury of cardiomyocytes using intracoronary cytoprotectors during endovascular reperfusion of the infarctrelated artery. J Intervent Cardiol. 2006;11:10–16.
Smilari L, La Mela C, Santagati A, et al. Study of left ventricular function in ischemic cardiomyopathies before and after phosphocreatine infusion. Echocardiographic study. Curr Ther Res. 1987;41:557–567.
Ferraro S, Codella C, Palumbo F, et al. Hemodynamic effects of creatine phosphate in patients with congestive heart failure: a doubleblind comparison trial versus placebo. Clin Cardiol. 1996;19:699–703.
Cafiero M, Strumia E, Pirone S, Pacileo S, Santoro R. Efficacia della creatina fosfato nel trattamento dei pazienti con insufficienza cardiaca, valutazione ecocardiografica dopo trattamento acuto e protratto. Clin Ter. 1994;144:321–328.
Strozzi C, Bagni B, Ferri A. Creatine phosphate in the treatment of chronic ischemic heart failure. Curr Ther Res. 1992;51:925–932.
Scattolin G, Gabellini A, Desideri A, et al. Diastolic function and creatine phosphate: an echocardiografic study. Curr Ther Res. 1993;54:562–571.
Gelfgat EB, Dalili IG, Shakhtakhtinskaya FN, Yagisarova NM, Shirinova EA. The effect of phosphocreatine on the tolerance of physical exercise in patients with ischemic heart disease. In: Saks VA, Bobkov YG, Strumia E, eds. Proceeding of the Symposium “Creatine Phosphate: Biochemistry, Pharmacology and Clinical Efficiency”, Baku, 6 October 1986. Torino: Minerva Medica; 1987:270.
Grazioli I, Strumia E. Terapia con creatina fosfato nel paziente con insufficienza cardiaca in fase di scompenso: studio policentrico. G Ital Ric Clin Ter. 1989;10:39–45.
Grazioli I, Melzi G, Strumia E. Multicentre controlled study of creatine phosphate in the treatment of heart failure. Curr Ther Res. 1992;52:271–280.
Andreev NA, Andreeva TN, Bichkov IV. Effect of creatine phosphate in congestive heart failure. Curr Ther Res. 1992;51:649–660.
Galyautdinov GS, Saks VA, Kots Y, Vdovenko LG. Clinical application of creatine phosphate in congestive heart failure, evaluation of general clinical efficiency. Il Cuore. 1993;10:185–193.
Wang FR, Zheng X. Effects of phosphocreatine on plasma brain natriuretic peptide level and left ventricular function in patients with heart failure. Liaoning: Affiliated Hospital, Chinese Medicine University. Published at PJCCPVD, August 2008;16:29–31.
Yang W, Tang L. Clinical analysis on the treatment of pediatric myocarditis by neoton. Beijing Med J. 2001;23:19–20.
Dal Monte A, Leonardi LM, Figura F, et al. Effetti dell’apporto esogeno di fosfocreatina sulla potenza muscolare umana. Gazz Med Ital. 1976;135:1–11.
Tegazzin V, Rossi M, Schiavon M, et al. Indagine sulla performance di ciclisti trattati e non trattati con fosfocreatina. Biol Med. 1991;13:121–135.
Vorobiev DV, Vetrova EG, Larina IM, Popova IA, Grigoriev AI. Energy substrates, hormone responses and glucocorticoid binding in lymphocytes during intense physical exercise in humans following phosphocreatine administration. Eur J Appl Physiol Occup Physiol. 1996;74:534–540.
Satolli F, Marchesi G. Creatine phosphate in the rehabilitation of patients with muscle hypotonotrophy of the lower extremity. Curr Ther Res. 1989;46:67–73.
Agnese L, Tarello M, Grazioli I. Efficacia della creatina fosfato nel trattamento della ipotonotrofia da non uso della muscolatura scheletrica. Ort e Traum Oggi. 1992;12:107–113.
Pirola V, Pisani L, Teruzzi P. Valutazione del recupero del trofismo muscolare in pazienti anziani con frattura di femore trattati con creatina fosfato e fisiokinesiterapia. Clin Ter. 1991;139:115–119.
Weber P, Vlašicovábrová R, Semrád B. Asset of creatine phosphate for cardiocerebral syndrome treatment in acute myocardial infarction in old age. Cas Lék Ces. 1992;134:53–56.
Skrivanek O, Kalvach P, Benetin J, et al. Creatine phosphate in the treatment of acute ischaemic stroke. Prakticky Lekar. 1995;75:7–8.
Bakala J, Kalita Z. SPECT findings in patients with acute stroke treated with NEOTON. Paper presented at: Proceedings of the “ 13th International Czech and Slovak Neurovascular Symposium”; June 8–9, 1995, Zlin, Czech Republic.
Zhang G, Luo W, Zheng J, He W. Clinical observation and blood gas changes after the treatment of neoton in neonates with moderate or severe hypoxic ischemic encephalopathy. Pediatr Emerg Med. 2004;11:397–398.
Author information
Authors and Affiliations
Corresponding author
Additional information
To view enhanced content go to www.advancesintherapy.com
Rights and permissions
About this article
Cite this article
Strumia, E., Pelliccia, F. & D’Ambrosio, G. Creatine Phosphate: Pharmacological and Clinical Perspectives. Adv Therapy 29, 99–123 (2012). https://doi.org/10.1007/s12325-011-0091-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-011-0091-4